The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs. Here I explore whether crystal structures reflect this effect.
Archive for the ‘crystal_structure_mining’ Category
Mesomeric resonance in substituted benzenes: a crystallographic reality check.
Wednesday, August 26th, 2015Previously, I showed how conjugation in dienes and diaryls can be visualised by inspecting bond lengths as a function of torsions. Here is another illustration, this time of the mesomeric resonance on a benzene ring induced by an electron donating substituent (an amino group) or an electron withdrawing substituent (cyano).
A visualisation of the effects of conjugation; dienes and biaryls.
Tuesday, August 25th, 2015Here is another exploration of simple chemical concepts using crystal structures. Consider a simple diene: how does the central C-C bond length respond to the torsion angle between the two C=C bonds?
Intermolecular atom-atom bonds in crystals? The O…O case.
Saturday, July 25th, 2015I recently followed this bloggers trail; link1 → link2 to arrive at this delightful short commentary on atom-atom bonds in crystals[1] by Jack Dunitz. Here he discusses that age-old question (to chemists), what is a bond? Even almost 100 years after Gilbert Lewis’ famous analysis,[2] we continue to ponder this question. Indeed, quite a debate on this topic broke out in a recent post here. My eye was caught by one example in Jack's article: "The close stacking of planar anions, as occurs in salts of croconic acid …far from producing a lowering of the crystal energy, this stacking interaction in itself leads to an increase by several thousand kJ mol−1 arising from Coulombic repulsion between the doubly negatively charged anions" I thought I might explore this point a bit further in this post.
References
- J.D. Dunitz, "Intermolecular atom–atom bonds in crystals?", IUCrJ, vol. 2, pp. 157-158, 2015. https://doi.org/10.1107/s2052252515002006
- G.N. Lewis, "THE ATOM AND THE MOLECULE.", Journal of the American Chemical Society, vol. 38, pp. 762-785, 1916. https://doi.org/10.1021/ja02261a002
The Bürgi–Dunitz angle revisited: a mystery?
Tuesday, May 12th, 2015The Bürgi–Dunitz angle is one of those memes that most students of organic chemistry remember. It hypothesizes the geometry of attack of a nucleophile on a trigonal unsaturated (sp2) carbon in a molecule such as ketone, aldehyde, ester, and amide carbonyl. Its value obviously depends on the exact system, but is generally taken to be in the range 105-107°. A very good test of this approach is to search the crystal structure database (this was how it was originally established[1]).
References
- H. B:urgi, J. Dunitz, J. Lehn, and G. Wipff, "Stereochemistry of reaction paths at carbonyl centres", Tetrahedron, vol. 30, pp. 1563-1572, 1974. https://doi.org/10.1016/s0040-4020(01)90678-7
A new way of exploring the directing influence of (electron donating) substituents on benzene.
Friday, April 17th, 2015The knowledge that substituents on a benzene ring direct an electrophile engaged in a ring substitution reaction according to whether they withdraw or donate electrons is very old.[1] Introductory organic chemistry tells us that electron donating substituents promote the ortho and para positions over the meta. Here I try to recover some of this information by searching crystal structures.
References
- H.E. Armstrong, "XXVIII.—An explanation of the laws which govern substitution in the case of benzenoid compounds", J. Chem. Soc., Trans., vol. 51, pp. 258-268, 1887. https://doi.org/10.1039/ct8875100258
Halogen bonds 4: The strongest (?) halogen bond.
Sunday, December 7th, 2014
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[1]. 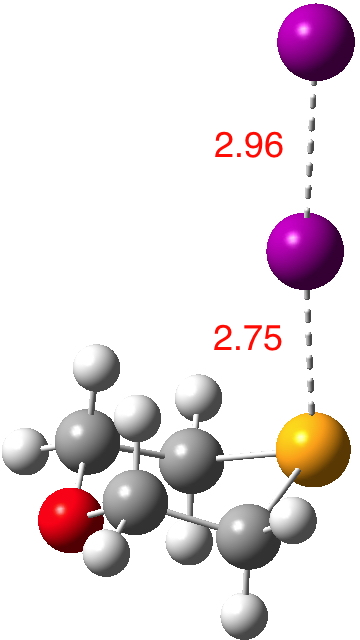 The features of interest include:
The features of interest include:
References
- H. Maddox, and J.D. McCullough, "The Crystal and Molecular Structure of the Iodine Complex of 1-Oxa-4-selenacyclohexane, C<sub>4</sub>H<sub>8</sub>OSe.I<sub>2</sub>", Inorganic Chemistry, vol. 5, pp. 522-526, 1966. https://doi.org/10.1021/ic50038a006
More simple experiments with crystal data. The pyramidalisation of nitrogen.
Saturday, November 1st, 2014We are approaching 1 million recorded crystal structures (actually, around 716,000 in the CCDC and just over 300,00 in COD). One delight with having this wealth of information is the simple little explorations that can take just a minute or so to do. This one was sparked by my helping a colleague update a set of interactive lecture demos dealing with stereochemistry. Three of the examples included molecules where chirality originates in stereogenic centres with just three attached groups. An example might be a sulfoxide, for which the priority rule is to assign the lone pair present with atomic number zero. The issue then arises as to whether this centre is configurationally stable, i.e. does it invert in an umbrella motion slowly or quickly. My initial intention was to see if crystal structures could cast any light at all on this aspect.
Halogen bonds: Part 1.
Saturday, November 29th, 2014Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
(more…)
Tags:crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »