Previously, I showed how conjugation in dienes and diaryls can be visualised by inspecting bond lengths as a function of torsions. Here is another illustration, this time of the mesomeric resonance on a benzene ring induced by an electron donating substituent (an amino group) or an electron withdrawing substituent (cyano).
In both cases, you can see this resonance showing as a lengthening of the C(ipso)-C(ortho) and C(meta)-C(para) bonds, and a contracting of the C(ortho)-C(meta) bonds. Does this reflect in the measured structures? The usual search is applied (R < 5%, no disorder, no errors) and qualified with the following:
- The amino has three bonds, and can bear either H, or 4-bonded carbon only.
- R on the ring can be either H or C.
- Three distances are defined.
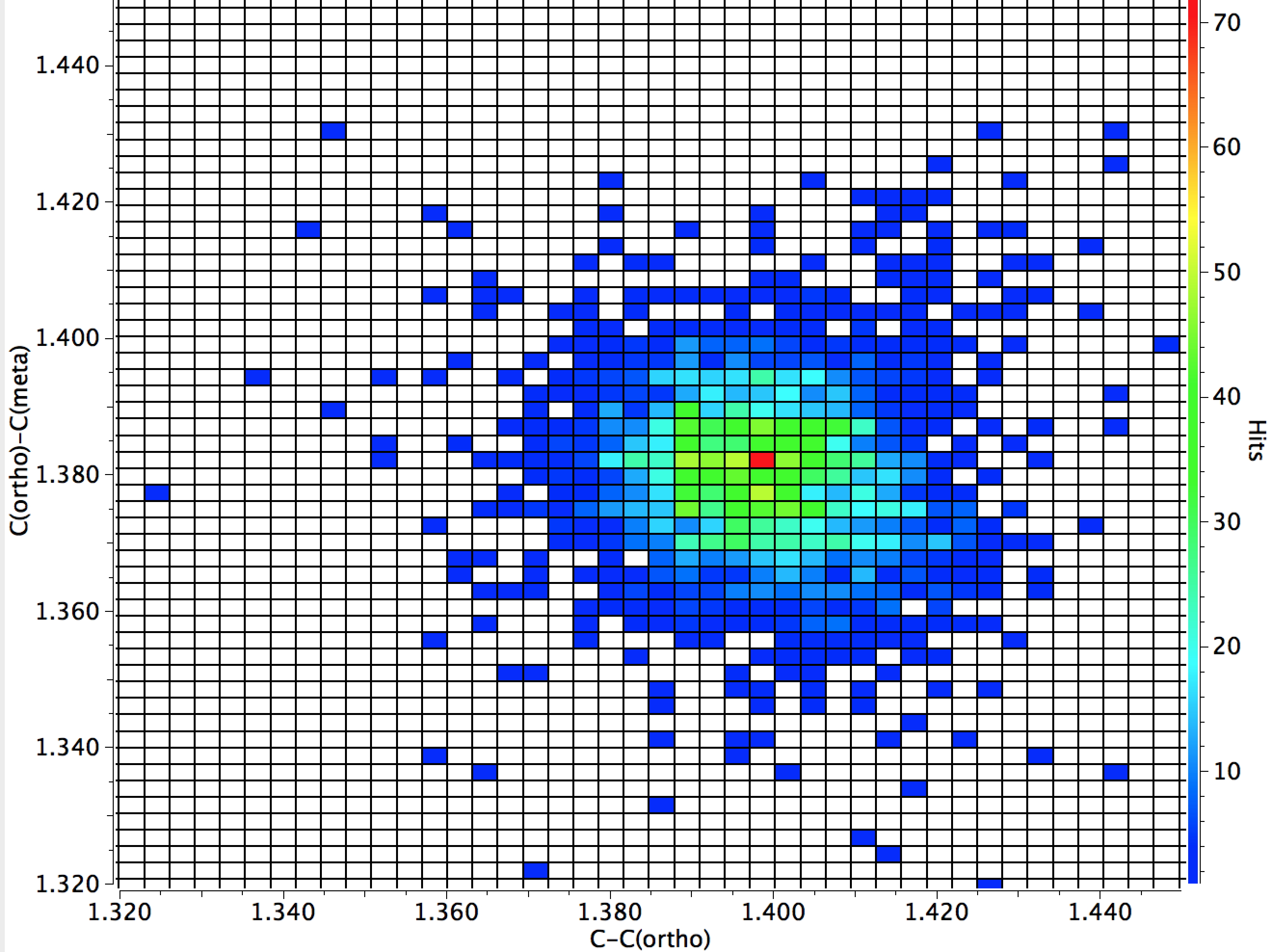
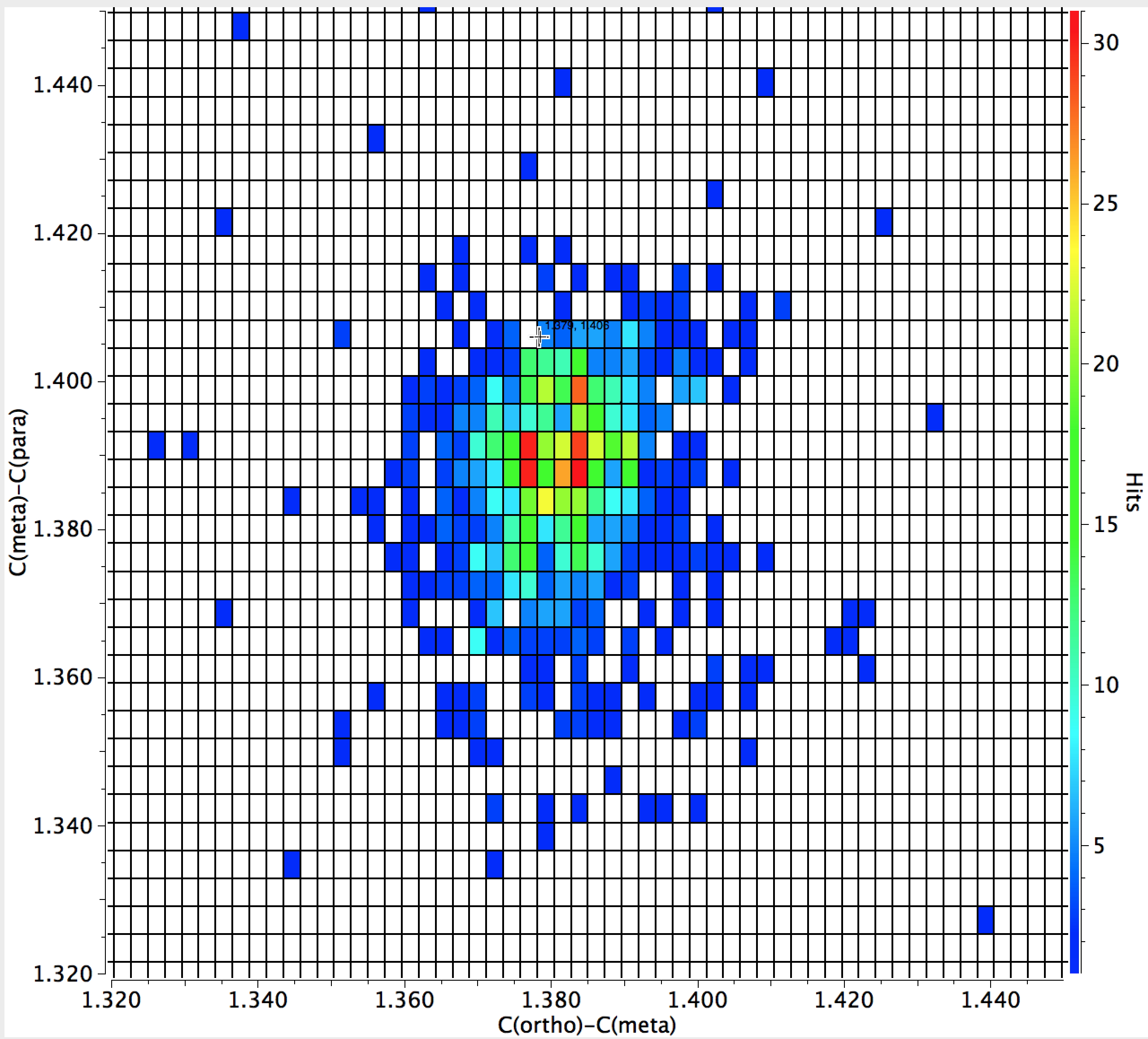
The results of a search are shown below; the hotspot shows the C-C(ortho) distance is close to 1.40Å, whilst the corresponding value for C(ortho)-C(meta) is 1.38Å, a contraction of ~0.02Å. The contraction is smaller for phenols (~0.01Å).
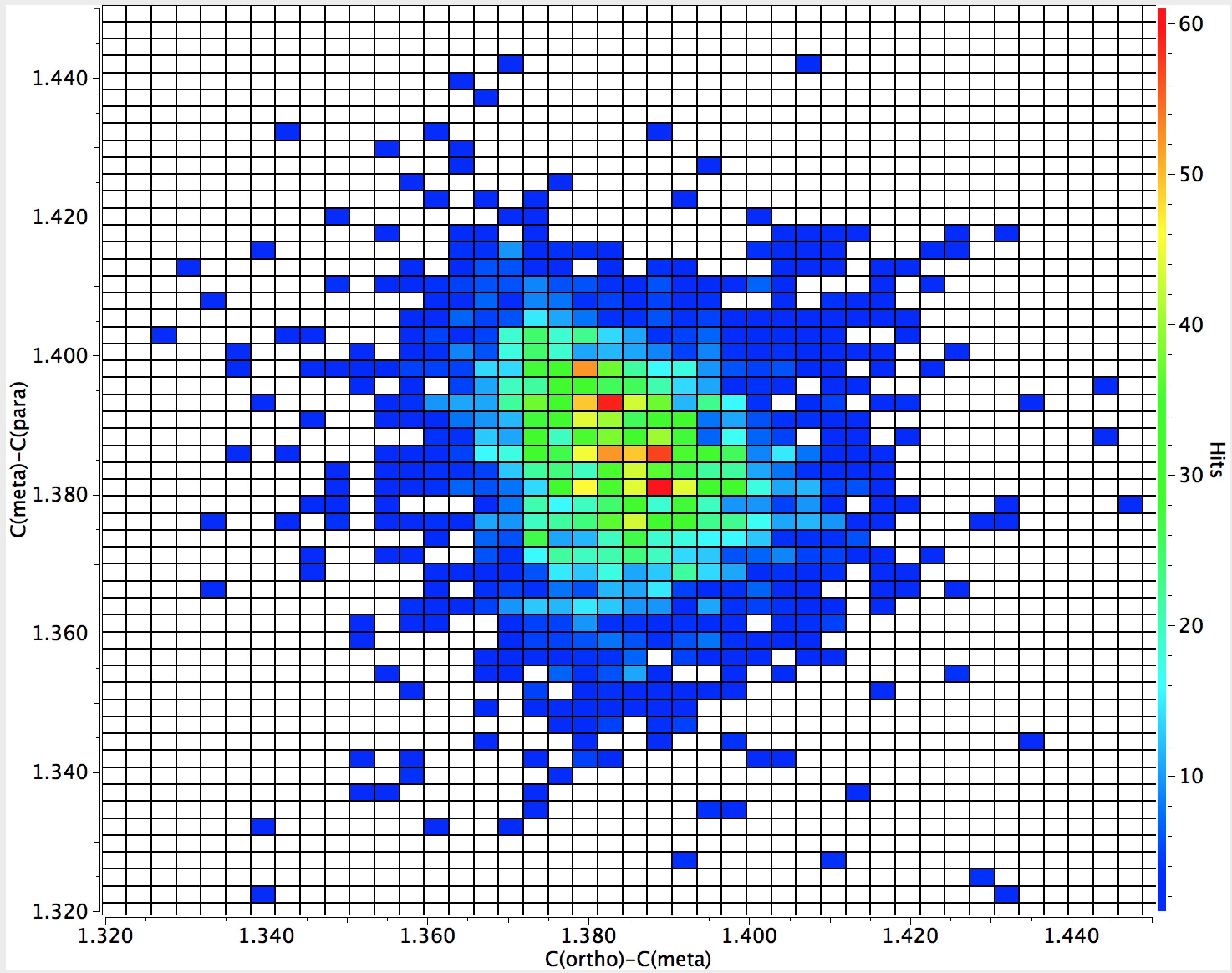
The C(ortho)-C(meta) vs C(meta)-C(para) amino plot shows a cluster of hotspots for which the former (1.38Å) is shorter than the latter (~1.39Å) but the effect is less clear cut as the distance from the substituent increases.
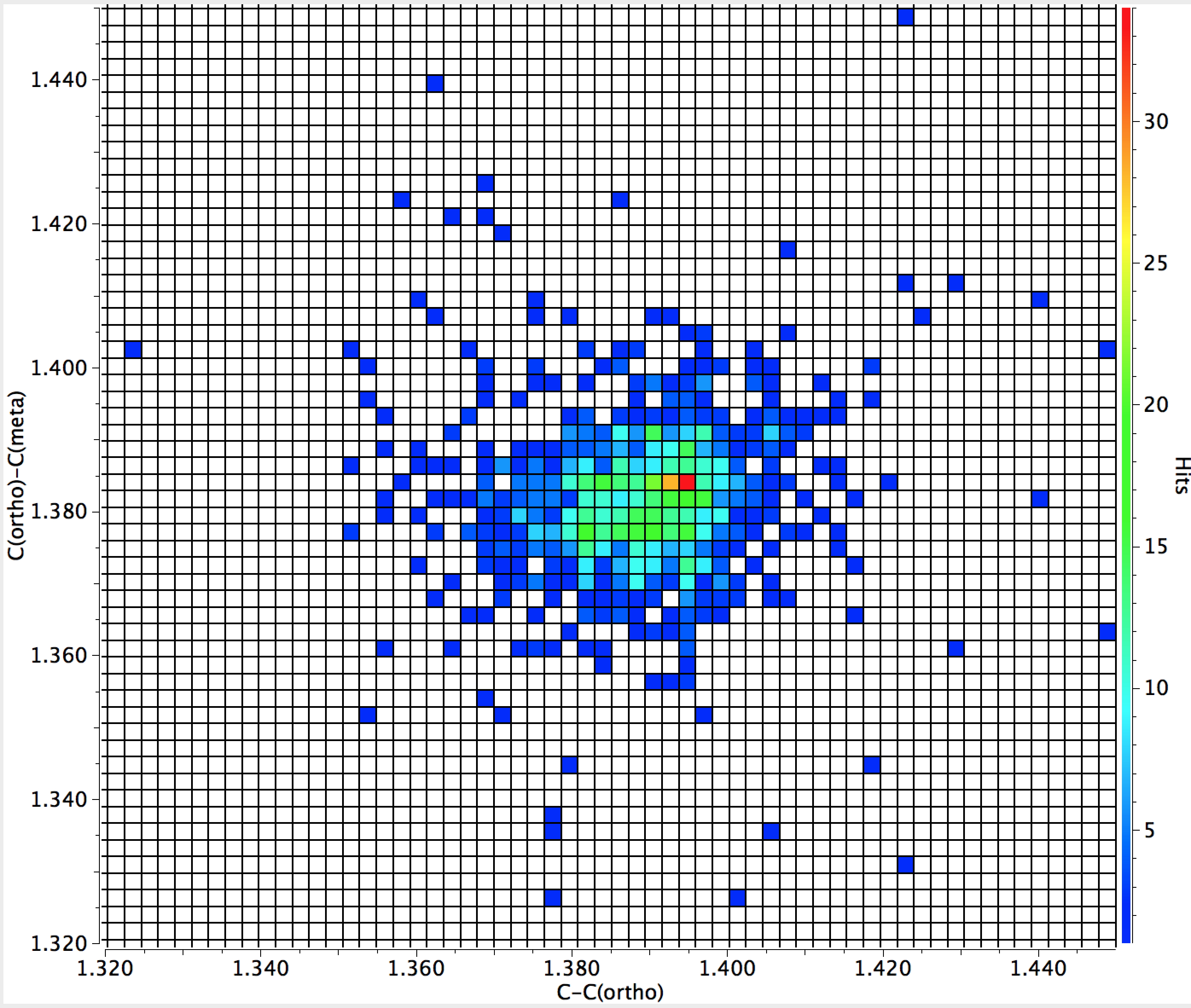
For an electron withdrawing cyano substituent, C(ipso)-C(ortho) at 1.395Å is longer than C(ortho)-C(meta) at 1.385Å, although the difference seems smaller than for the amino substituent. The (ortho)-C(meta) to C(meta)-C(para) comparison is similar.
These searches take but a few minutes to perform, and do serve as a reality check on the oft-seen mesomeric π-resonance shown in all organic text books.
Tags: chemical bonding, Chemical nomenclature, Hammett equation, Mesomeric effect, Substituent, Substitution reactions, usual search