Linear free energy relationships (LFER) are associated with the dawn of physical organic chemistry in the late 1930s and its objectives in understanding chemical reactivity as measured by reaction rates and equilibria.
Archive for the ‘reaction mechanism’ Category
Free energy relationships and their linearity: a test example.
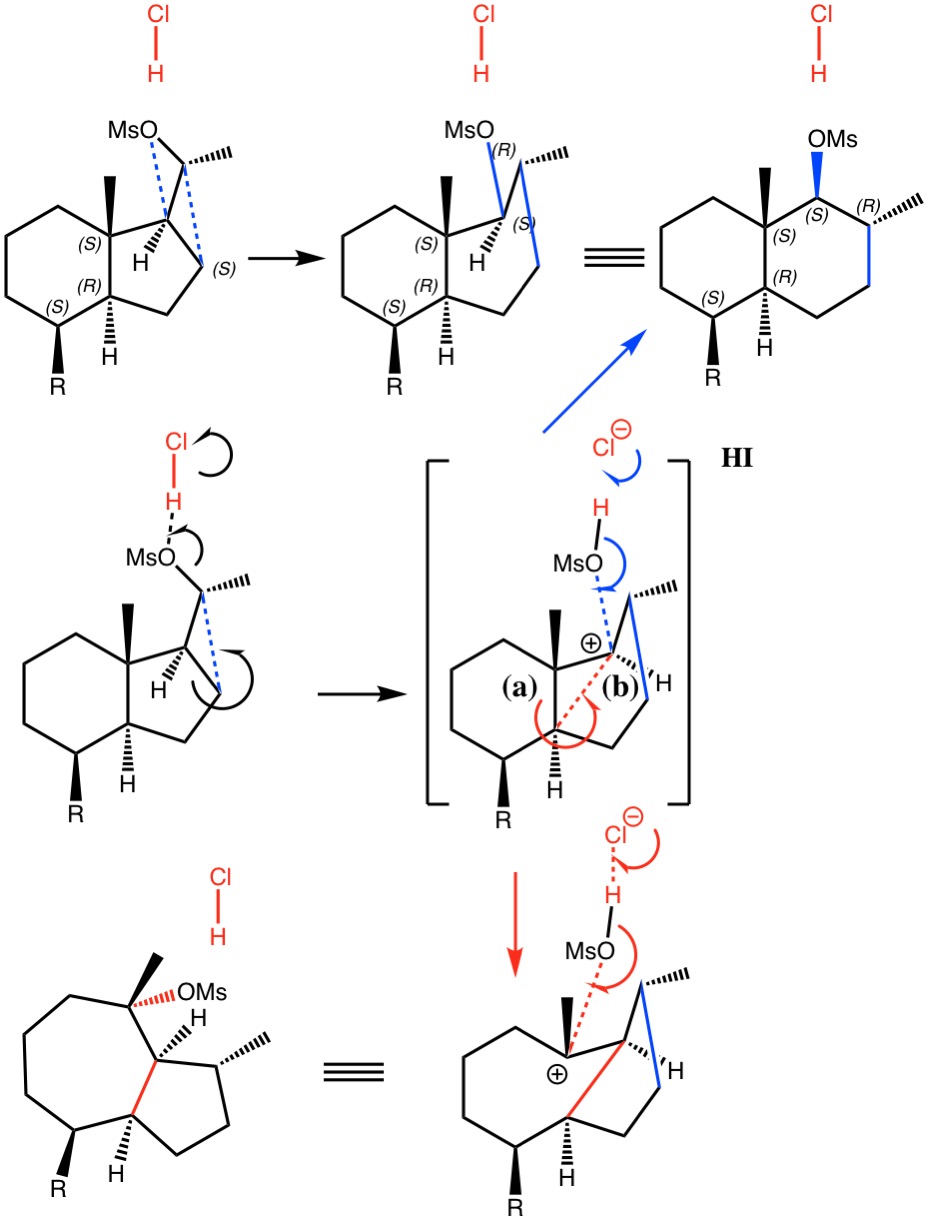
Sunday, January 13th, 2019Dyotropic Ring Expansion: more mechanistic reality checks.
Sunday, October 1st, 2017I noted in my WATOC conference report a presentation describing the use of calculated reaction barriers (and derived rate constants) as mechanistic reality checks. Computations, it was claimed, have now reached a level of accuracy whereby a barrier calculated as being 6 kcal/mol too high can start ringing mechanistic alarm bells. So when I came across this article[1] in which calculated barriers for a dyotropic ring expansion observed under mild conditions in dichloromethane as solvent were used to make mechanistic inferences, I decided to explore the mechanism a bit further.
References
- H. Santalla, O.N. Faza, G. Gómez, Y. Fall, and C. Silva López, "From Hydrindane to Decalin: A Mild Transformation through a Dyotropic Ring Expansion", Organic Letters, vol. 19, pp. 3648-3651, 2017. https://doi.org/10.1021/acs.orglett.7b01621
The conformation of enols: revealed and explained.
Thursday, April 6th, 2017Enols are simple compounds with an OH group as a substituent on a C=C double bond and with a very distinct conformational preference for the OH group. Here I take a look at this preference as revealed by crystal structures, with the theoretical explanation.
What is the (calculated) structure of a norbornyl cation anion-pair in water?
Saturday, April 1st, 2017In a comment appended to an earlier post, I mused about the magnitude of the force constant relating to the interconversion between a classical and a non-classical structure for the norbornyl cation. Most calculations indicate the force constant for an “isolated” symmetrical cation is +ve, which means it is a true minimum and not a transition state for a [1,2] shift. The latter would have been required if the species equilibrated between two classical carbocations. I then pondered what might happen to both the magnitude and the sign of this force constant if various layers of solvation and eventually a counter-ion were to be applied to the molecule, so that a bridge of sorts between the different states of solid crystals, superacid and aqueous solutions might be built.
Reaction coordinates vs Dynamic trajectories as illustrated by an example reaction mechanism.
Monday, March 20th, 2017The example a few posts back of how methane might invert its configuration by transposing two hydrogen atoms illustrated the reaction mechanism by locating a transition state and following it down in energy using an intrinsic reaction coordinate (IRC). Here I explore an alternative method based instead on computing a molecular dynamics trajectory (MD).
How does silane invert (its configuration)?
Thursday, March 16th, 2017In the previous post, I found intriguing the mechanism by which methane (CH4) inverts by transposing two of its hydrogens. Here I take a look at silane, SiH4.
How does methane invert (its configuration)?
Thursday, March 16th, 2017This is a spin-off from the table I constructed here for further chemical examples of the classical/non-classical norbornyl cation conundrum. One possible entry would include the transition state for inversion of methane via a square planar geometry as compared with e.g. NiH4 for which the square planar motif is its minimum. So is square planar methane a true transition state for inversion (of configuration) of carbon?
Forming a stabilized m-benzyne.
Friday, January 20th, 2017The story so far. Inspired by the report of the most polar neutral compound yet made, I suggested some candidates based on the azulene ring system that if made might be even more polar. This then led to considering a smaller π-analogue of azulene, m-benzyne. Here I ponder how a derivative of this molecule might be made, using computational profiling as one reality check.