Author Archive
Saturday, October 8th, 2022
Sometimes you come across a reaction which is so simple in concept that you wonder why it took so long to be accomplished in practice. In this case, replacing toxic ozone O3 as used to fragment an alkene into two carbonyl compounds (“ozonolysis”) by a relatively non-toxic simple nitro-group based reagent, ArNO2 in which the central atom of ozone is substituted by an N-aryl group. As reported by Derek Lowe, two groups have published[1], [2] details of such a reaction (Ar = 4-cyano or 3-CF3,5-NO2). But there are (at least) two tricks; the first is to use photo-excitation using purple LEDs (390nm light) to activate the nitro group. The second is to establish the best aryl substituents to use for achieving maximum yields of the carbonyl compounds and the best conditions for achieving the cyclo-reversion reaction, shown below as TS1. That step requires heating the cyclo-adduct up to ~80° in (aqueous) acetonitrile for anywhere between 1-48 hours. Here I take a computational look at that last step, the premise being that if such a model is available for this mechanism, it could in principle be used to optimise the conditions for the process.
(more…)
References
- D.E. Wise, E.S. Gogarnoiu, A.D. Duke, J.M. Paolillo, T.L. Vacala, W.A. Hussain, and M. Parasram, "Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes", Journal of the American Chemical Society, vol. 144, pp. 15437-15442, 2022. https://doi.org/10.1021/jacs.2c05648
- A. Ruffoni, C. Hampton, M. Simonetti, and D. Leonori, "Photoexcited nitroarenes for the oxidative cleavage of alkenes", Nature, vol. 610, pp. 81-86, 2022. https://doi.org/10.1038/s41586-022-05211-0
Tags:Interesting chemistry
Posted in reaction mechanism | No Comments »
Friday, September 30th, 2022
The term bispericyclic reaction was famously coined by Caramella et al in 2002[1] to describe the unusual features of the apparently innocuous dimerisation of cyclopentadiene. It shows features of two paths for different pericyclic reactions, comprising a 2+4 cycloaddition in the early stages, but evolving into a (degenerate) pair of [3,3] sigmatropic reactions in the latter stages. Houk (who also uses the term ambimodal) has in recent years extended the number of examples of such pericyclic sequences to trispericyclic[2] (see here) and even an ambimodel tetrapericyclic reaction, as reported at the recent WATOC event. Here I show an example of a new type of bispericyclic reaction, comprising a 2+4 cycloaddition combined with a electrocyclic ring opening.
(more…)
References
- P. Caramella, P. Quadrelli, and L. Toma, "An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the <i>E</i><i>ndo</i> Dimerization of Cyclopentadiene", Journal of the American Chemical Society, vol. 124, pp. 1130-1131, 2002. https://doi.org/10.1021/ja016622h
- X. Xue, C.S. Jamieson, M. Garcia-Borràs, X. Dong, Z. Yang, and K.N. Houk, "Ambimodal Trispericyclic Transition State and Dynamic Control of Periselectivity", Journal of the American Chemical Society, vol. 141, pp. 1217-1221, 2019. https://doi.org/10.1021/jacs.8b12674
Posted in reaction mechanism | No Comments »
Thursday, September 15th, 2022
In previously asking what the largest angle subtended at four-coordinate carbon might be, I noted that as the angle increases beyond 180°, the carbon becomes inverted, or hemispherical (all four ligands in one hemisphere). So what does a search for this situation reveal in the CSD? The query can be formulated as below, in which the distance from the centroid of the four ligands to the central carbon is specified to be in e.g. the range 0.8 to 1.1Å. For tetrahedral carbon surrounded by four carbon ligands, the value would be close to zero, so any value larger than say 0.8Å is worth inspecting.
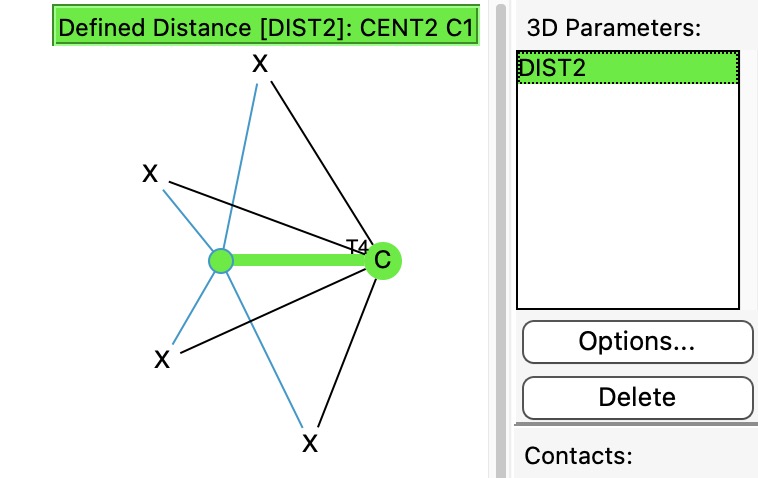
(more…)
Posted in Uncategorised | 2 Comments »
Sunday, September 11th, 2022
Four-coordinate carbon normally adopts a tetrahedral shape, where the four angles at the carbon are all 109.47°. But how large can that angle get, and can it even get to be 180°?
(more…)
Tags:Interesting chemistry
Posted in Uncategorised | 1 Comment »
Thursday, September 8th, 2022
The recently reported synthesis[1] of octafluorocubane established a sublimation point as 168.1–177.1°C (a melting point was not observed). In contrast, the heavier perfluoro-octane has an m.p. of -25°C. Why the difference? Firstly, the crystal structure is shown below, albeit as a dimer rather than a periodic lattice (click on image to obtain 3D coordinates).
(more…)
References
- M. Sugiyama, M. Akiyama, Y. Yonezawa, K. Komaguchi, M. Higashi, K. Nozaki, and T. Okazoe, "Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor", Science, vol. 377, pp. 756-759, 2022. https://doi.org/10.1126/science.abq0516
Posted in Uncategorised | 2 Comments »
Monday, August 29th, 2022
Derek Lowe reports the story[1] that the recently synthesized octafluorocubane can absorb one electron to form a radical anion – an electron in a cube. So I thought it would be fun to compute exactly where that electron sits!
(more…)
References
- M. Sugiyama, M. Akiyama, Y. Yonezawa, K. Komaguchi, M. Higashi, K. Nozaki, and T. Okazoe, "Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor", Science, vol. 377, pp. 756-759, 2022. https://doi.org/10.1126/science.abq0516
Posted in Uncategorised | 10 Comments »
Thursday, August 25th, 2022
A previous post was triggered by Peter alerting me that interactive electronic supporting information (IESI) we had submitted to a journal in 2005[1] appeared to be strangely missing from the article landing page. This set me off recollecting our journey, which had started around 1998, and to explore what the current state of these ancient IESIs were in 2022. I have now reached 2014 in this journey, which is being recorded as it happens in the comments page of the post. I discovered there were four distinct stages in that evolution of IESI which I thought it would be of interest to record here.
(more…)
References
- H.S. Rzepa, and M.E. Cass, "A Computational Study of the Nondissociative Mechanisms that Interchange Apical and Equatorial Atoms in Square Pyramidal Molecules", Inorganic Chemistry, vol. 45, pp. 3958-3963, 2006. https://doi.org/10.1021/ic0519988
Posted in Uncategorised | No Comments »
Wednesday, August 17th, 2022
Previously, I looked at autocatalytic mechanisms where the carboxyl group of an oxetane-carboxylic acid could catalyse its transformation to a lactone, finding that a chain of two such groups were required to achieve the result. Here I look at an alternative mode where the oxetane-carboxylate itself acts as the transfer chain, via a H-bonded dimer shown below.
(more…)
Posted in reaction mechanism | No Comments »
Sunday, August 14th, 2022
Having established a viable model for the unexpected isomerism of oxetane carboxylic acids to lactones[1], and taken a look at a variation in the proton transfer catalyst needed to accomplish the transformation, I now investigate the substrate itself.
(more…)
References
- B. Chalyk, A. Grynyova, K. Filimonova, T.V. Rudenko, D. Dibchak, and P.K. Mykhailiuk, "Unexpected Isomerization of Oxetane-Carboxylic Acids", Organic Letters, vol. 24, pp. 4722-4728, 2022. https://doi.org/10.1021/acs.orglett.2c01402
Posted in reaction mechanism | 1 Comment »
Saturday, August 13th, 2022
Previously, a mechanism with a reasonable predicted energy was modelled for the isomerisation of an oxetane carboxylic acid to a lactone by using two further molecules of acid to transfer the proton and in the process encouraging an Sn2 reaction with inversion to open the oxetane ring. 
(more…)
Tags:Interesting chemistry
Posted in reaction mechanism | No Comments »
