Science is about making connections. And these can often be made between the most unlikely concepts. Thus in the posts I have made about pentavalent carbon, one can identify a series of conceptual connections. The first, by Matthias Bickelhaupt and co, resulted in the suggestion of a possible frozen SN2 transition state. They used astatine, and this enabled a connection to be made between another good nucleophile/nucleofuge, cyclopentadienyl anion. This too seems to lead to a frozen Sn2 transition state. The cyclopentadienyl theme then asks whether this anion can coordinate a much simpler unit, a C2+ dication (rather than Bickelhaupt’s suggestion of a (NC)3C+ cation/radical) and indeed that complex is also frozen, again with 5-coordinate carbon, and this time with five equal C-C bonds. So here, the perhaps inevitable progression of ideas moves on to examining the properties of this complex, the outcome being a quite counter-intuitive suggestion which moves us into new territory.
Author Archive
It’s Hexa-coordinate carbon Spock – but not as we know it!
Friday, October 2nd, 2009It’s penta-coordinate carbon Spock- but not as we know it!
Wednesday, September 30th, 2009In the previous two posts, I noted the recent suggestion of how a stable frozen SN2 transition state might be made. This is characterised by a central carbon with five coordinated ligands. The original suggestion included two astatine atoms as ligands (X=At), but in my post I suggested an alternative which would have five carbon ligands instead (X=cyclopentadienyl anion).
Capturing penta-coordinate carbon! (Part 2).
Wednesday, September 23rd, 2009In this follow-up to the previous post, I will try to address the question what is the nature of the bonds in penta-coordinate carbon?
Capturing penta-coordinate carbon! (Part 1).
Tuesday, September 22nd, 2009The bimolecular nucleophilic substitution reaction at saturated carbon is an icon of organic chemistry, and is better known by its mechanistic label, SN2. It is normally a slow reaction, with half lives often measured in hours. This implies a significant barrier to reaction (~15-20 kcal/mol) for the transition state, shown below (X is normally both a good nucleophile and a good nucleofuge/leaving group, such as halide, cyanide, etc. Y can have a wide variety of forms).
Spotting the unexpected: Anomeric effects
Friday, September 18th, 2009Chemistry can be very focussed nowadays. This especially applies to target-driven synthesis, where the objective is to make a specified molecule, in perhaps as an original manner as possible. A welcome, but not always essential aspect of such syntheses is the discovery of new chemistry. In this blog, I will suggest that the focus on the target can mean that interesting chemistry can get over-looked (or if observed, not fully exploited in subsequent publications). Taking a synthesis-oriented publication at (almost) random entitled Synthesis of 1-Oxadecalins from Anisole Promoted by Tungsten (DOI: 10.1021/ja803605m) which appeared in 2008, the following molecule appears as one of the (many) intermediates.
The Fragile Web
Monday, August 31st, 2009One of the many clever things that clever people can do with the Web is harvest it, aggregate it, classify it etc. Its not just Google that does this sort of thing! Egon Willighagen is one of those clever people. He runs the Chemical blogspace which does all sorts of amazing things with blogs.
Towards the ultimate bond!
Monday, August 24th, 2009
The 100th anniversary of G. N. Lewis’ famous electron pair theory of bonding is rapidly approaching in 2016 (DOI: 10.1021/ja02261a002). He set out a theory of bond types ranging from 1-6 electrons. The strongest bond recognized by this theory was the 6-electron triple bond, a good example of which occurs in dinitrogen, N2. In terms of valence electrons, nitrogen has an atomic configuration of 2s2, 2p3. Each atom has five electrons in total, some or all of which in principle could be used for forming bonds. An exploration of this motif across the entire periodic table is presented in part one of this blog.
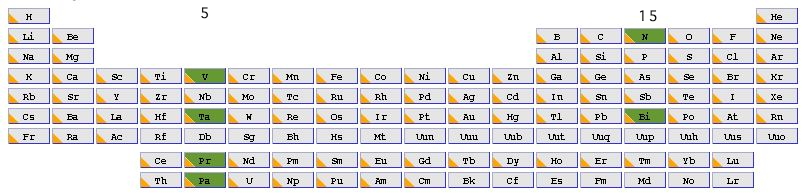
Nitrogen is in the main group 15, and the element at the bottom of this group is Bismuth (also with the same atomic configuration). We can then move to the corresponding column of the transition series, this time occupying group 5. The first examplar in this set, Vanadium has an atomic configuration of 3d3, 4s2, again five valence electrons, but now utilizing the d- rather than the p-shell of valence atomic orbitals (AOs). The final forage across the period table would land us with Pr and Pa, which occupy the lanthanide and actinide series respectively, and which have atomic configurations of 4f3, 6s2 and 5f2, 6d1 and 7s2 respectively. You can now see the theme developing; how does the bonding develop between two atoms that between them have ten valence electrons occupying molecular orbitals constructed from s, and then either p, d or f atomic orbitals. The next in that series, g atomic orbitals, are thought unlikely to have any chemical significance in the presently known periodic table.
Molecular toys: Tetrahedral cavities
Saturday, July 4th, 2009
An earlier post described how a (spherical) halide anion fitted snugly into a cavity generated by the simple molecule propanone, itself assembled by sodium cations coordinating to the oxygen. A recent elaboration of this theme, reminiscent of the children’s toys where objects have to be fitted into the only cavity that matches their shape, Nitschke and co-workers report the creation of a molecule with a tetrahedral rather than a spherical cavity (DOI: 10.1126/science.1175313 ), into which another but much smaller tetrahedral molecule is fitted. The small molecule is P4, in which each of the three valencies of the P atom is directed to a corner of the tetrahedron. The large molecule comprises four Fe atoms. These are each octahedrally coordinated with six ligand sites, three of which mimic the P atoms in also being directed towards the remaining three vertices of a tetrahedron.
Longer is stronger.
Saturday, June 6th, 2009The iconic diagram below represents a cornerstone of organic chemistry. Generations of chemists have learnt early on in their studies of the subject that these two representations of where the electron pairs in benzene might be located (formally called electronic resonance or valence bond forms) each contribute ~50% to the overall wavefunction, and that the real electronic description is in effect an average of these two (that is the implied meaning of the double headed arrow). This means that the six C-C bonds in benzene must all be of equal length. The diagrams, everyone knows, do not mean that benzene has three short and three long C-C bonds.