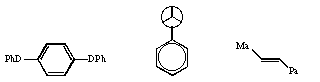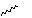Practice Problems in Pericyclic Reactions.
The following are a set of practice problems in Pericyclic Mechanisms,
prepared by Henry Rzepa for the for a second year course in Pericyclic Reactions at the
Department of Chemistry, Imperial College.
Each of these problems is intended to take between 1-3 hours to solve.
They are NOT typical of examination questions! Please note also that the compound numbers are not consecutive between questions.
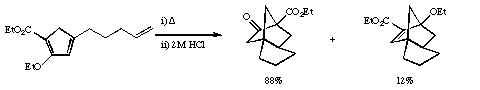
Qu 11. a) Propose a mechanism for the following transformation, including an explanation for the formation of two products and indicating the number of electrons involved in each step. Extrapolating from this explanation, suggest a related third product that might have formed, but as it happens was not detected with these particular substituents.
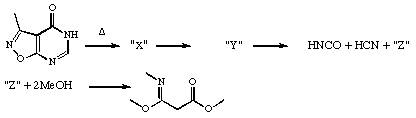
b) In the following sequence, the technique of flash vacuum pyrolysis results in the trapping of HNCO, HCN and a novel third product Z on a cold finger. The species Z shows at least one ir band in the region 2280 cm-1. When trapped with two quivalents of methanol, Z gives the product shown. Suggest possible identities for the two intermediates ³X and Y as well as Z, and mechanisms for all the reactions, including details of nomenclature for each step.
Answer
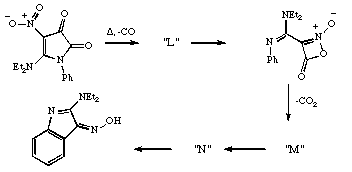
Qu 12. In the following sequence, suggest possible structures for the intermediates L, M and N, together with mechanisms and nomenclature for their formation. Discuss whether each step is formally allowed by the pericyclic selection rules for thermal reactions, or whether it requires the presence of traces of acid or base.
Answer
Qu 13. Suggest a nomenclature for the following three substrates;
Answer
Qu 14. When a mixture of hexa-2,4-dienol, CS2, KOH and prop-2-ynyl bromide is stirred for 12 hours at room temperature, the expected compound 1 is not isolated. Instead an oil "A" with the following spectroscopic properties is obtained; νmax 1646 cm-1, δH 1.70 (3H, d, J 6Hz), 2.23 (1H, t, J 3Hz), 3.73 (2H, d, J 3Hz), 4.84 (1H, dd, J 7, 8 Hz), 5.16 (1H, d, J 10 Hz), 5.27 (1H, d, J 17 Hz), 5.51 (1H, dd, J 8, 16.5 Hz), 5.72 (1H, dq, J 6, 16.5 Hz), 5.88 (1H, ddd, J 7, 10, 17 Hz). On heating to 180°C, compound 2 was isolated in 62% yield, via the presumed intermediate shown. Identify compound "A", suggest mechanisms for the subsequent reactions of 1, and assign the spectroscopic data, including a discussion of the coupling constants. In compound 2, suggest a probable stereochemical assignment for the methyl group marked with a
Answer
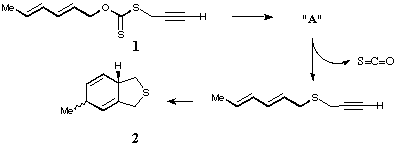
Qu 15. The 1,3 elimination of HCl from the imidoyl chloride isomers shown proceeds rapidly at 0*C to give the two compounds 3 and 4. On standing at room temperature, these rearrange to "X" or "Y", for which the following spectral data are obtained. X: δH 0.56 (3H, d, J 6.2 Hz), 2.40 (1H, dq, J 9.8, 6.2 Hz), 2.85 (1H, d, J 9.8 Hz), 8.51 (1H, s), 7.18-7.55 (9H, m); δC 5 (q), 15 (d), 33 (d), 54 (s), 155 (s) + aromatics. Y: δH 1.08 (3H, d, J 6.2 Hz), 0.49 (1H, dq, J 5.5, 6.2 Hz), 2.66 (1H, d, J 5.5 Hz), 8.16 (1H, s), 7.25-7.55 (9H, m); δC 28 (q), 14 (d), 32 (d), 58 (s), 153 (s) + aromatics. On refluxing in benzene, either "X" or "Y" interconvert via the presumed intermediacy of compound 5, which in turn slowly isomerises to 6, the final isolated product. Suggest structures for "X" and "Y" based on spectral and mechanistic evidence and propose likely mechanisms for their formation and subsequent reactions, paying particular attention to the stereochemistry of the species involved. (Hints: Try drawing alternative resonance forms of 3 and 4, and pay particular regard to the differences in the chemical shifts of X and Y. The multiplicities of the carbon nmr signals are those obtained with off-resonance decoupling).
Answer
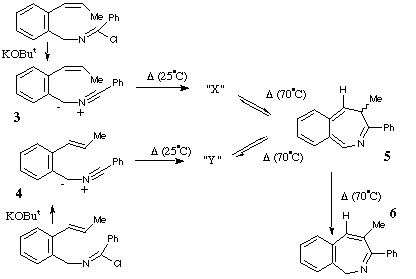
Qu 16. The synthesis of the naturally occuring coumarin ester Gravelliferone is accomplished by heating the compound [1] in the presence of weak base. Two intermediates [2] and [3] are thought to be implicated in the mechanism. Suggest a possible structure for [2] and mechanisms for the overall transformation, indicating any steps that might involve the base.
Answer
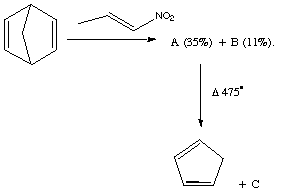
Qu 17. 1-Nitropropene when added slowly to a solution of phenyl isocyanate and triethylamine (which together act as a dehydrating reagent) in the presence of norbornadiene forms a mixture of two isomeric compounds A and B. These both show three similar vinylic nmr peaks, but are distinguished by different couplings in the non-vinylic region. A: δ3.45 (J 8, 1.5 Hz), and 4.82 (J 8, 1.3 Hz) and B: δ3.87 (J 9.5, 4.2 Hz), and 5.29 (J 9.5, 4.3 Hz). Flash vacuum pyrolysis of A+B yields cyclopentadiene and a new compound C, the latter having the following 1H nmr spectrum: δ 5.61 (J 10.9, 0.9Hz), 5.90 (J 17.8, 0.9 Hz), 6.47 (J 1.7Hz), 6.8 (J 17.8, 10.9 Hz), 8.32 (J 1.7 Hz), which includes three vinylic and two aromatic resonances, and one surprisingly low ortho aromatic coupling. Suggest structures for A, B and C, mechanisms for their formation and an interpretation of the different couplings manifested for A and B (Hint: try using the MacroModel modelling program).
Answer
Qu 18. Random dot autostereograms are a way of representing three dimensional images. Does this compound contain any chiral centres?
Answer
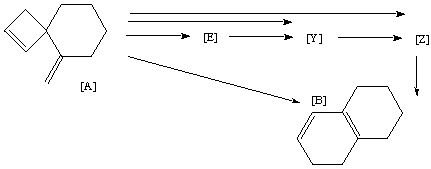
Qu. 19. The transformation of [A] to [B] by heating has been extensively studied kinetically. The evidence seems to suggest that direct conversion does not occur. Synthesis of the triene [Z] suggests it is converted to [B] very rapidly. Mechanisms have been proposed which indicate that whilst direct conversion of [A] to [Z] is possible, it cannot be the exclusive pathway, since an intermediate [E] is detected during reaction. [E] cannot convert thermally to [Z] at the temperatures used, but would have to go via [Y]. It is also possible that [Y] too is formed directly from [A], but this is thought less likely on steric grounds.
Suggest possible structures for [E], [Y], [Z], mechanisms for all the possible conversion routes shown with arrows below, and explain the cause of the steric grounds referred to above.
Answer
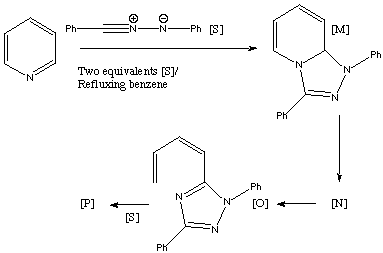
Qu. 20. Reaction of two equivalents of the [1,3] dipolar species [S] with pyridine gives a 43% yield of the adduct [P]. The 1H NMR of [P] is δ 3.15 (dd, J 17, 6.8Hz), 4.00 (dd, J 17, 11.5), 6.20 (dd, J 11.5, 8.5), 6.42 (dd, J 11.5, 1.2), 6.45 (multiplet, J 11.5, 8.5, 6.8, 1.2), 6.8-8.4 (20H, aromatics). Three intermediates [M], [N and [O] are thought to be involved. Suggest possible structures for [N] and [P], together with mechanisms for their formation, assignment of the NMR data for [P] and any stereochemical conclusions derived from this data.
Answer
© Henry S. Rzepa, 1978-2014. Hide|show Toolbar.