Practice Problems in Pericyclic Reactions.
The following are a set of practice problems in Pericyclic Mechanisms,
prepared by Henry Rzepa for the for a second year course in Pericyclic Reactions at the
Department of Chemistry, Imperial College.
Each of these problems is intended to take between 1-3 hours to solve.
They are NOT typical of examination questions! Please note also that the compound numbers are not consecutive between questions.
Qu 11. a)
Y. Himeda, K. Hiratani, M. Hatanaka and I. Ueda, J. Chem. Soc., Chem. Commun, 1992, 1684. b)

T. Mosandl, C. O. Kappe, R. Flammang and C. Wentrup, J. Chem. Soc., Chem. Commun, 1992, 1571.
Back to Problems
Qu 12,
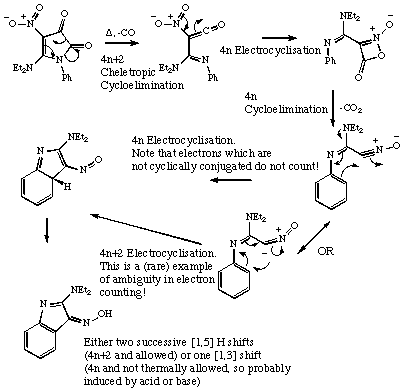
C. O. Kappe, G. Kollenz and C. Wentrup, J. Chem. Soc., Chem. Commun, 1992, 485.
Back to Problems
Qu 13.
Back to Problems
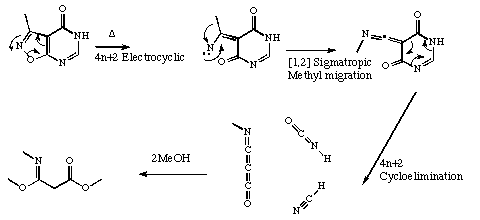
Qu 14. See K. Harano, M. Eto, K. Ono, K. Misaka and T. Hisano, J. Chem. Soc., Perkin Trans 1, 1993, 299 for further details.
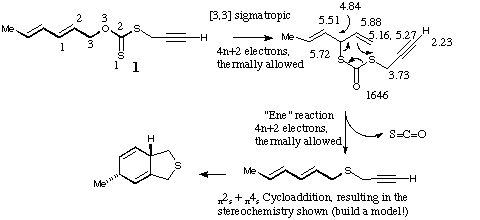
Back to Problems
Qu 15. Species 3 and 4 appear to react as carbenes! The two isomers X and Y are distinguished by the different chemical shift values of the Me and H groups induced by the adjacent aromatic ring (anisotropy). The observation that both X and Y can be isolated eliminates the possibility that 5 is formed directly from 3 or 4 by a 4n electron electrocyclisation (assuming the inversion barrier in 5 is small!). Another possibility of 3 or 4 acting as 1,3 dipoles and adding directly to the alkene to give X or Y is eliminated by spectral evidence and the necessity of the resulting cyclobutene having to ring open with disrotation (Huckel ts) to give 5, a process not favoured by the selection rules. See K R Motion, I. R. Robertson, J. T. Sharp and M. D. Walkinshaw, J. Chem,. Soc., Perkin Trans 1, 1992, 1709 for further details.
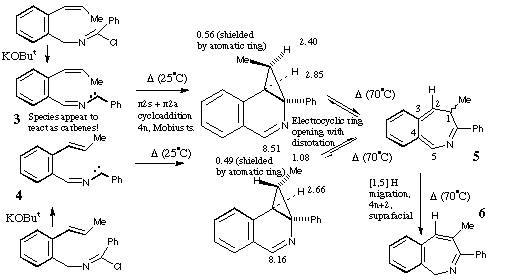
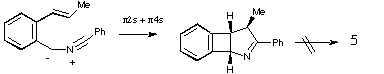
Back to Problems
Qu 16 N. Cairns, L. M. Harwood and D. P. Astles, J. Chem. Soc., Perkin Trans 1, 1994, 3101, propose a so called "tandem" series of [3,3] sigmatropic rearrangements, also known as Claisen rearrangements, for the synthesis of a series of natural coumarins.
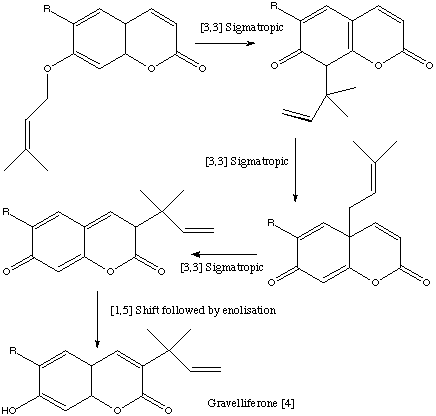
Back to Problems
Qu 17 P. W. Ambler, R. M. Paton and J. M. Trout, J. Chem. Soc., Chem. Commun., 1994, 2661, report the use of norbornadiene as an acetylene equivalent. The two adducts A and B are exo and endo isomers, which are distinguished by different 3J couplings, as predicted by the Karplus relationship. Molecular models show that the vicinal angle in the exo isomer (~ 75*) is close to the minimum in the Karplus function, whereas the endo isomer (~ 40*) should exhibit larger couplings. The angle of ~ 0* for the vicinal bridgehead protons leads to a medium sized coupling. The ortho coupling in the isoxazole product is surprisingly small, although it is in fact typical for such systems.

Back to Problems
Qu 18 Random dot autostereograms are restricted to representing images in a series of planes. It is not possible to represent continuously varying depth. The structure here is Gravelliferone (really!).
Back to Problems
Qu 19
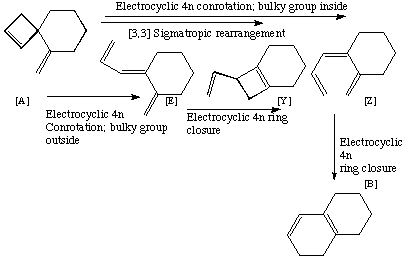
Back to Problems
Qu 20
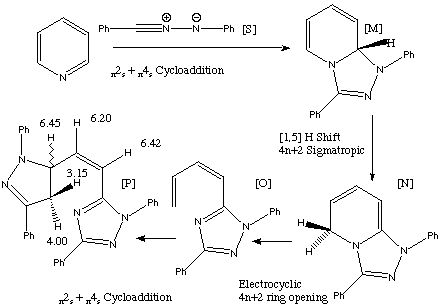
The abnormally high chemical shift of the 5-H proton at 6.45 ppm originates in part from the adjacency to N (which defines the chemospecificity) and in part from the 3D structure of the molecule, which places it in the (deshielding) plane of the other heterocyclic ring. To rotate this model, click here. Back to Problems
© Henry S. Rzepa, 1978-2014. Hide|show Toolbar.