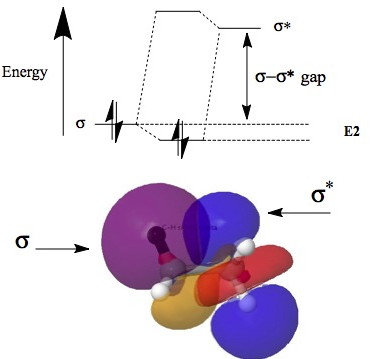
 A form of theory known as second-order perturbation theory shows how the pair of electrons in such a σ-orbital interacts with the vacant σ*-orbital orbital. The result is stabilization of the filled bonding NBO (by an energy E(2)) and destabilization (by an energy >E(2)) of the antibonding NBO. Since the latter has no electrons, the overall effect is of stabilization, but only if
A form of theory known as second-order perturbation theory shows how the pair of electrons in such a σ-orbital interacts with the vacant σ*-orbital orbital. The result is stabilization of the filled bonding NBO (by an energy E(2)) and destabilization (by an energy >E(2)) of the antibonding NBO. Since the latter has no electrons, the overall effect is of stabilization, but only if
- if the σ-σ* energy gap is sufficiently small
- the two orbitals can overlap efficiently. This overlap is optimal for the anti-periplanar orientation.
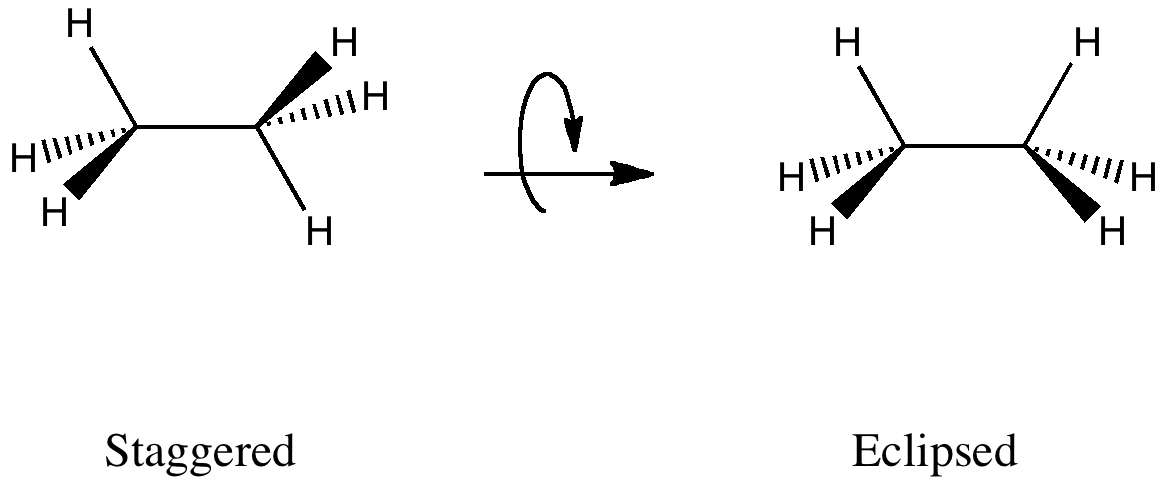
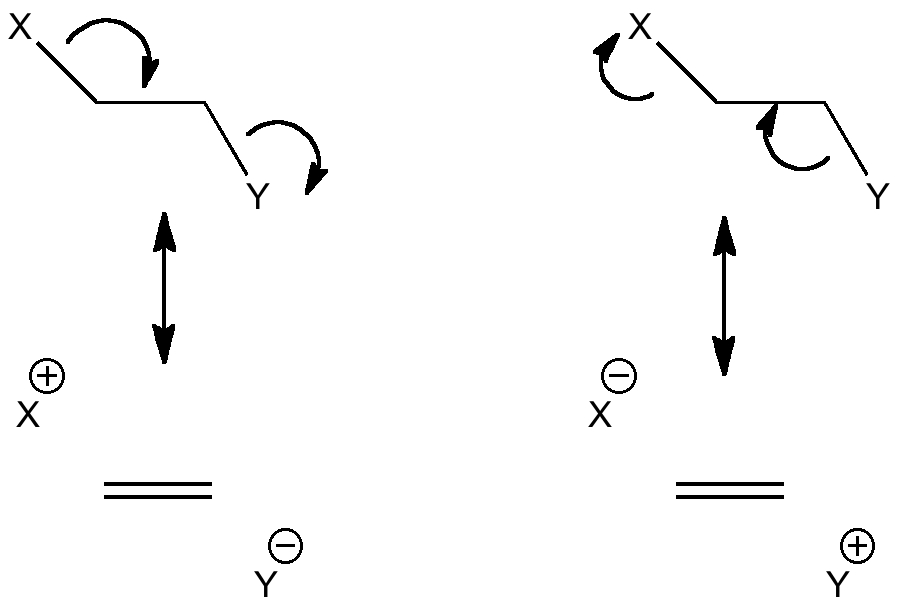
This effect can be represented using "arrow pushing" as conjugation of the following type (X=Y=H for ethane):
In staggered ethane, there are six (equivalent) anti-periplanar interactions between pairs of a σC-H -orbital and a σ*C-H -orbital (X=Y=H), for each of which E(2) = 3.2 kcal/mol.
These anti-periplanar orientations are all missing in the eclipsed orientation, being replaced by syn-periplanar interactions for which E(2) has the smaller value of 1.3 kcal/mol each due to reduced overlap.
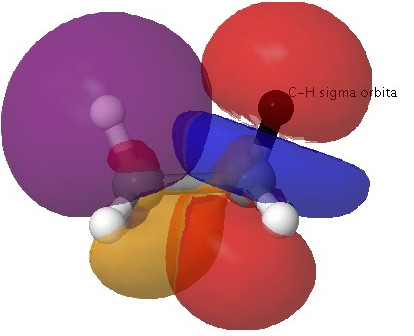
Effect two: Bond-bond or Pauli repulsions. In a similar manner, one can show that the interaction between two occupied C-H σ-orbitals results in overall destabilization (the top orbital being destabilized by more than the bottom is stabilized).
- Thus the total pairwise (steric) repulsions in eclipsed ethane comprise three pairs of eclipsed C-H bonds and six pairs of C-H bonds oriented at 120°; +22.3 kcal/mol (for details, see here).
- In staggered ethane, three anti-periplanar and six C-H pairs oriented at 60° totalling +21.9 kcal/mol
Overall, these favour the staggered form (by 0.4 kcal/mol). Whilst the effect is relatively small in ethane, as molecules get larger, it mounts up! In general however, effect one is more stabilizing than effect two is destabilizing.
Effect three: van der Waals (dispersion) forces. Whereas effects 1 and 2 operate at relatively short (bonded) internuclear distances a rather different one operates at longer (non-bonded) inter-atomic distances:
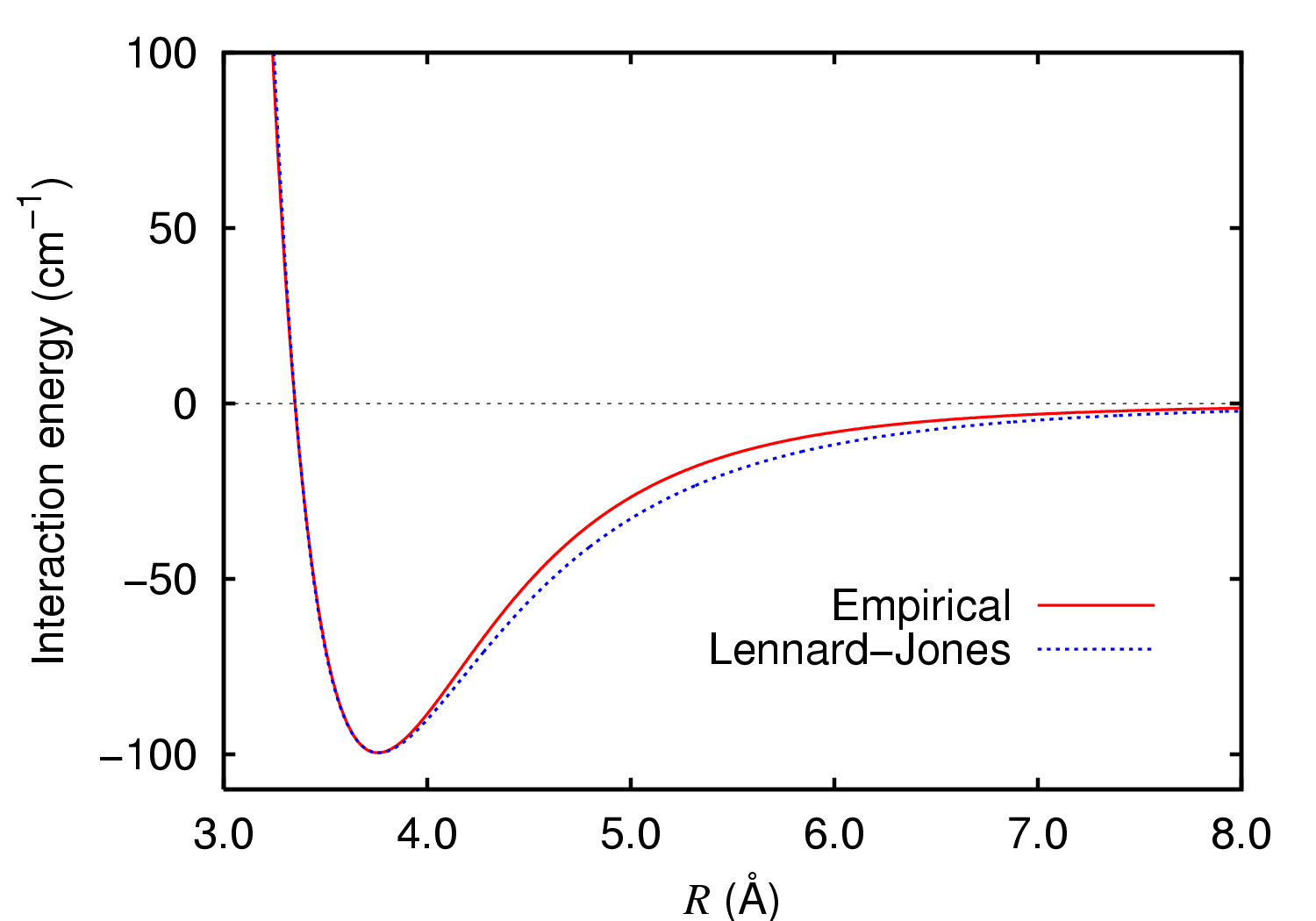
At short distances the function is strongly repulsive (see also 2 above), but at longer distances it actually becomes attractive (which is why longer chain alkanes tend to be liquids and not gases, vis petrol!). The maximum attraction occurs at a distance corresponding to the sum of the so-called van der Waals radii for the two atoms concerned. For argon, this radius is 2*1.88Å =3.76Å (above, 100 cm-1 ~0.3 kcal/mol or ~1.3 kJ/mol). For hydrogen, that radius is 1.2Å (when interacting with other hydrogens) or 1.1Å (when interacting with other elements, see DOI: 10.1021/jp8111556, 10.1002/chem.200800987, 10.1524/zkri.2009.1158). In typical organic compounds, this means that maximum attraction occurs at the following distances for typical pairs of non-bonded atoms:

- H...H; 2.4Å (becoming repulsive at ~<2.1Å unless it's a dihydrogen bond).
- C...H; 2.7Å
- N...H; 2.58Å
- O...H; 2.62Å
- Cl...H; 2.85Å
- F...F; 2.9Å
- C...C; 3.2Å
- Cl...Cl; 3.5Å
- Br...Br; 3.9Å
- "Me"..."Me"; 4.0Å
- I...I; 4.3Å