 .
.As the rings get larger, the conformational complexity starts to increase. A cyclo-octane ring now has six conformational minima but only two of these have significant populations at room temperatures, a boat-chair and a crown (or this unusual example).
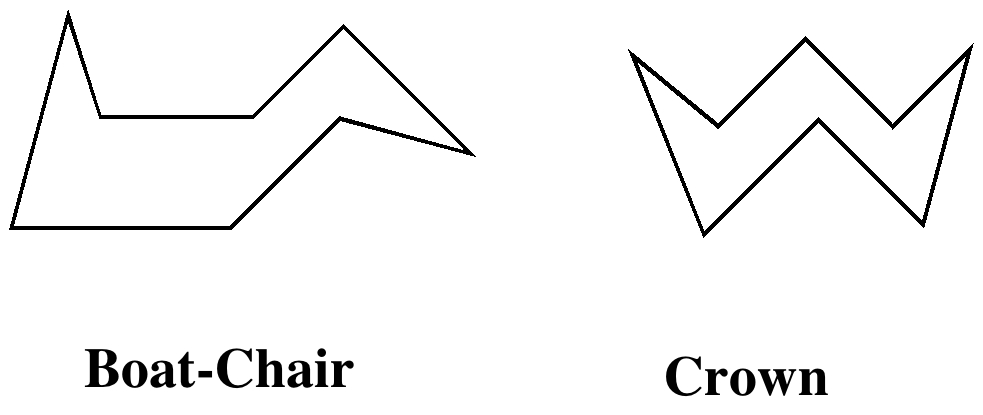
Neither of these conformations has entirely optimal bond angles or twist angles however, and so these medium sized rings are somewhat strained. There are also so-called transannular contacts between hydrogens across the ring that may be van der Waals attractive (H...H ~ 2.1-2.2Å) along with anti-periplanar relations which can mediate reactions (beyond the scope of this lecture). Many natural products have rings this size or larger.  .
.
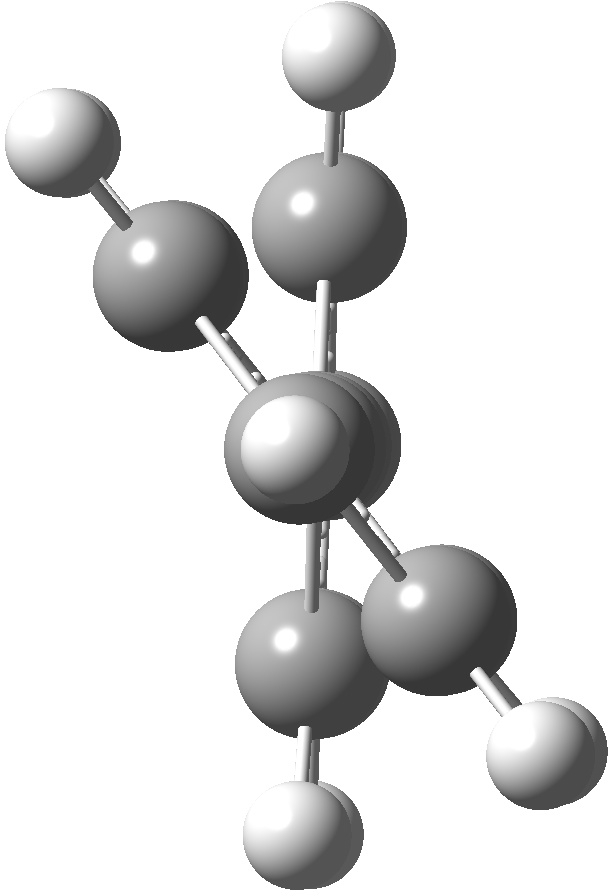
Before we leave the cycloalkanes, here is the crystal structure of cyclo-C24H48 which combines the features of the longer chain acyclic isomers (i.e. the gauche conformation leading to short H...H van der Waals attractive contacts) and the chair-like motif of the cycloalkanes, but also with something of a frustrated Gecko motif!
The is the smallest sized ring which can accomodate a trans-alkene into the ring system (trans-cycloheptene is very unstable, isomerising to the cis-alkene at room temperatures by rotation about the C=C bond, DOI: 10.1021/ja055388i). These systems are of interest because they introduce a new conformational phenomenon, that of atropisomerism.
Definition of atropisomerism: Atropisomers are stereoisomers resulting from hindered rotation about one or more single bonds, where the energy barrier to rotation is high enough to allow for the isolation of the conformers. This normally means that the conformers should interconvert with a half-life of ~103s at a given temperature; at 300K this implies a barrier of ~22 kcal/mol.
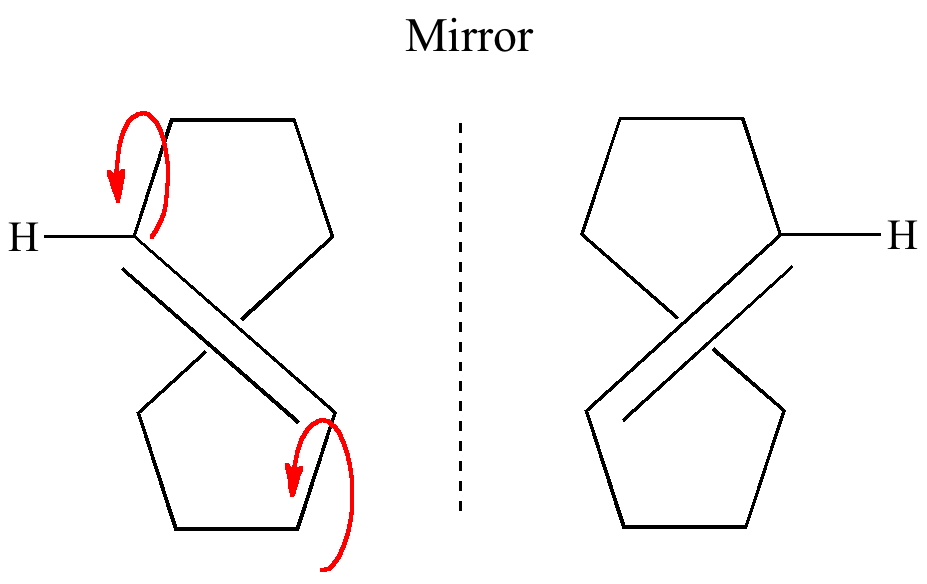
Trans-Cyclo-octene itself is disymmetric, having only a C2 axis of symmetry, ( X-ray) and it is therefore not superimposable upon its mirror image (its optical rotation, [α]D ~464°). So we can see that atropisomerism is a special case of a conformational isomer also being a configurational isomer (the boundary between these two is ~ the half-life as defined above).
In fact, the two enantiomers can be separated by forming a diastereomeric complex with a chiral platinum salt (DOI: 10.1021/ja00903a049)
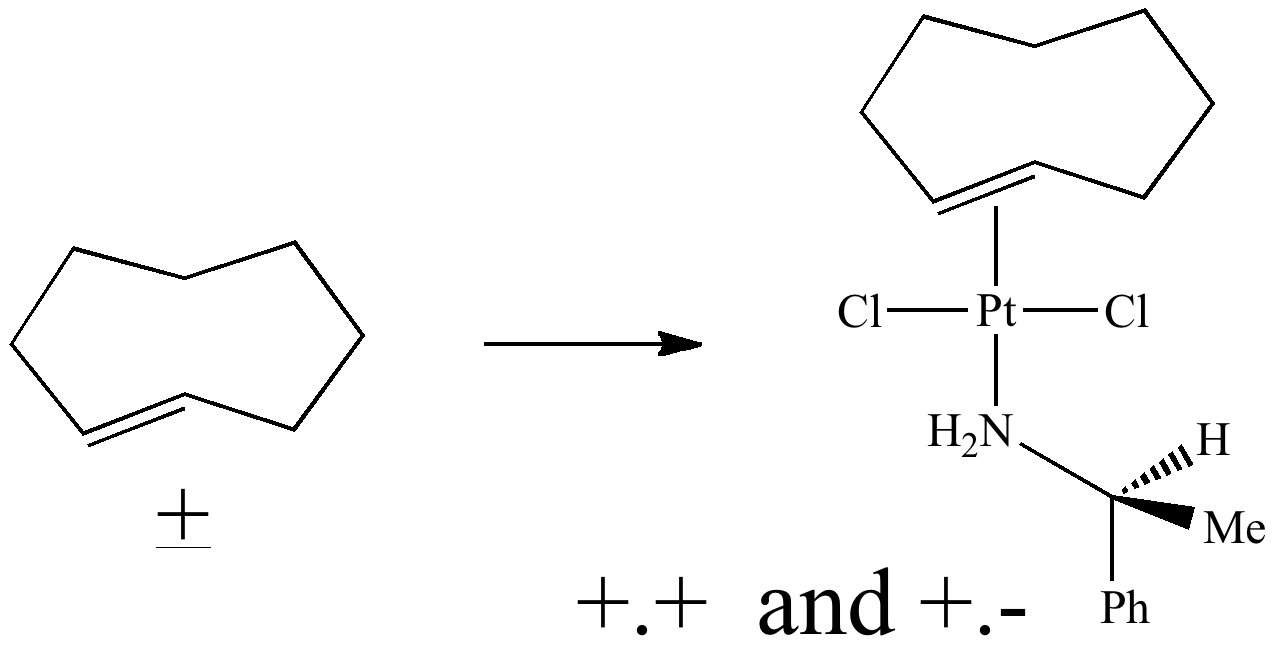
Once separated, the:
This enantiomerisation occurs by simultaneously rotating about the two single bonds shown in red (above), which involves an alkene hydrogen rotating around to point into the ring itself. The smaller the ring, the greater the transannular (repulsive) contacts there will be, and the more the rest of the ring will have to distort to reduce them.
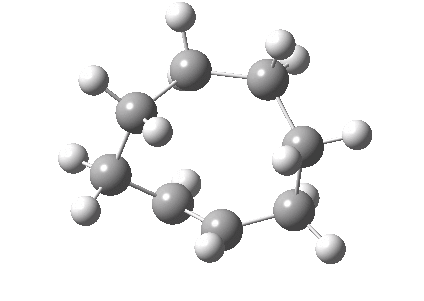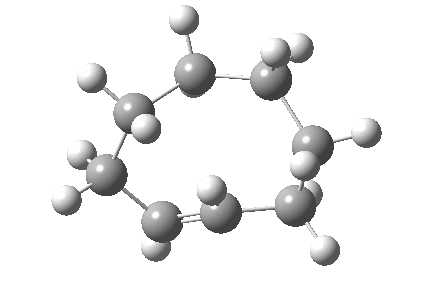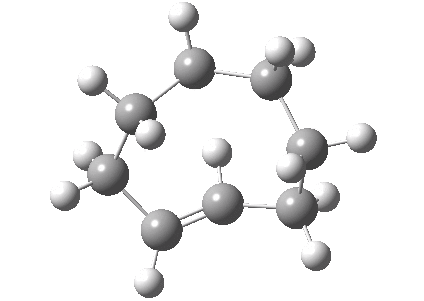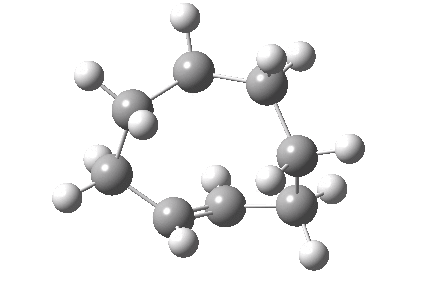
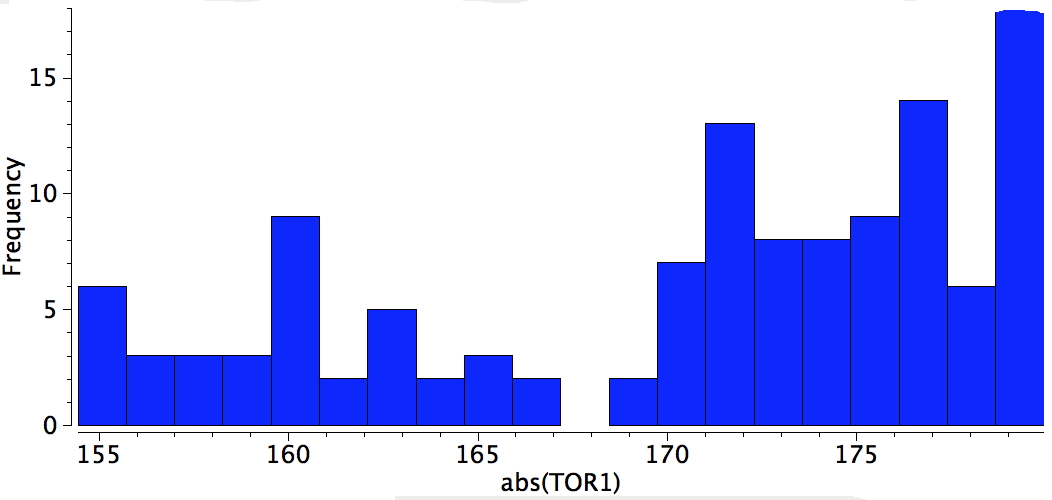
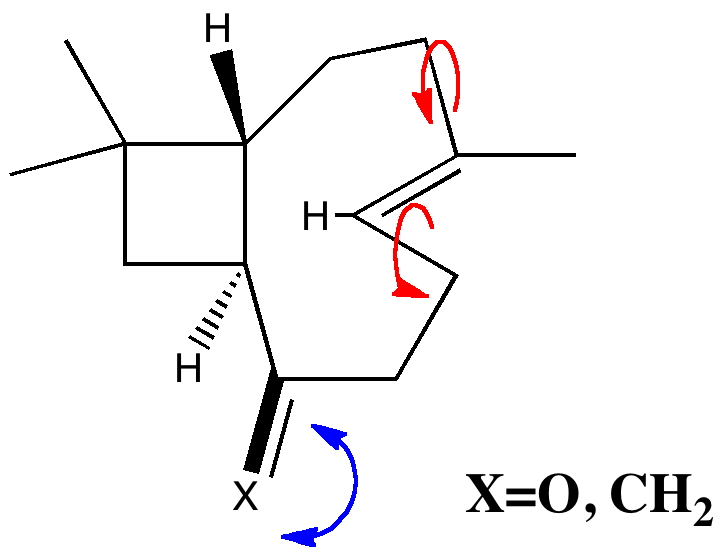
An example of a natural product which exhibits this behaviour is caryophyllene, which contains a trans-alkene in a 9-membered ring and which can exhibit atropisomerism not only across the alkene but also the carbonyl group! This latter introduces the concept of conformational atropisomerism resulting from angular strain (seen also in the natural product Taxol).
Here we are concerned not with molecules that have an asymmetric carbon but rather with those which are asymmetric by virtue of their shape alone, and whose enantiomeric conformers may be interconverted simply by rotation about a single C-C single bond. In order that the enantiomers may be separated at room temperature the barrier to rotation must be reasonably high (> 20 kcal/mol, e.g. trans-cyclononene). Examples of acyclic systems which can be so induced to restrict rotation about a single bond include biphenyls and binaphthyls (the latter, when one aryl ring is naphthyl is a very valuable ligand for inducing asymmetric catalysis).
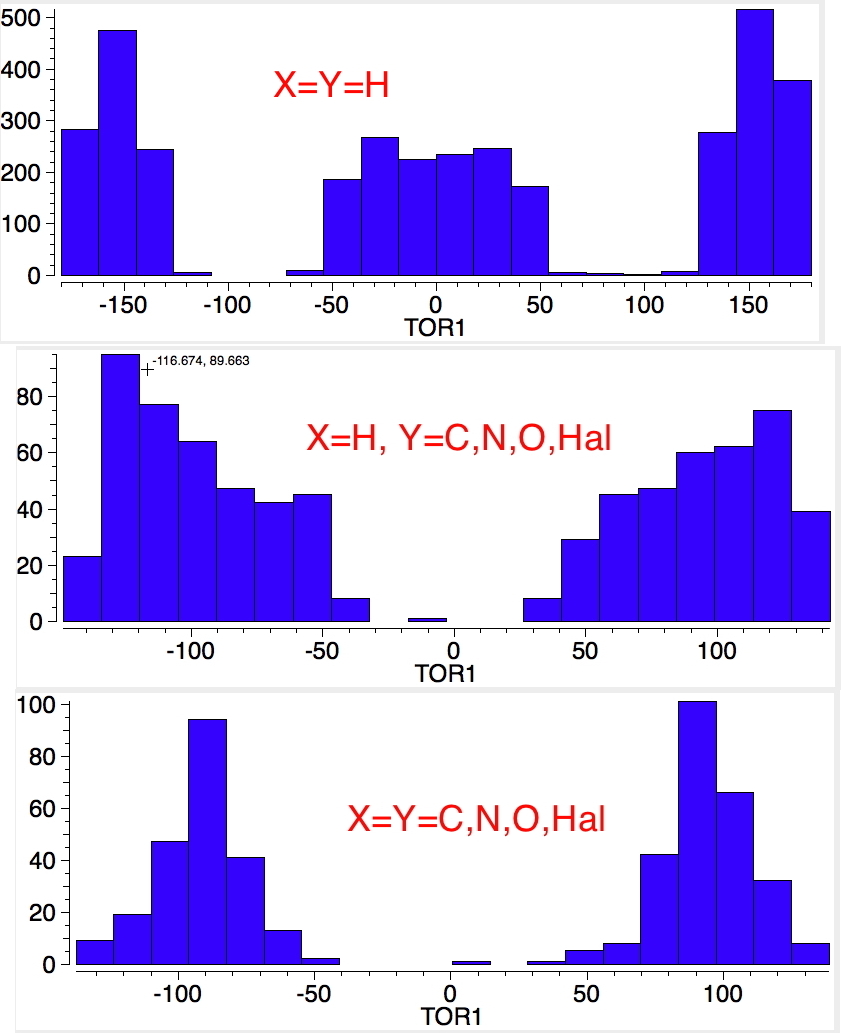
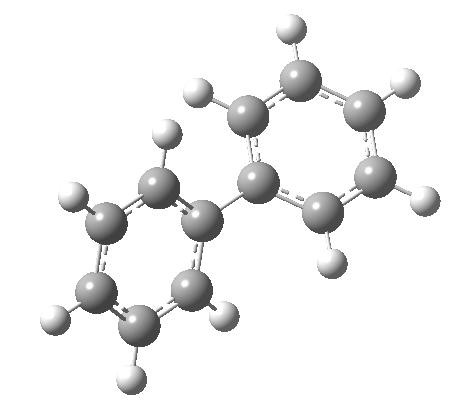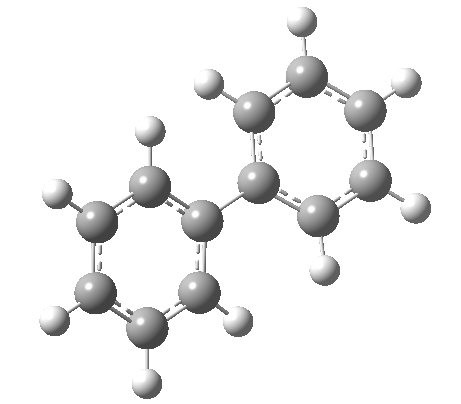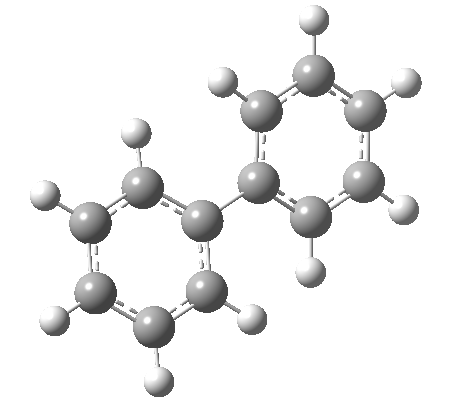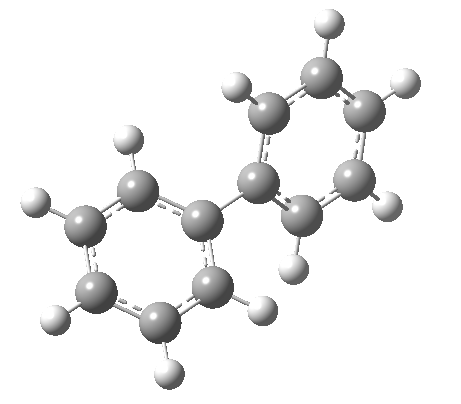
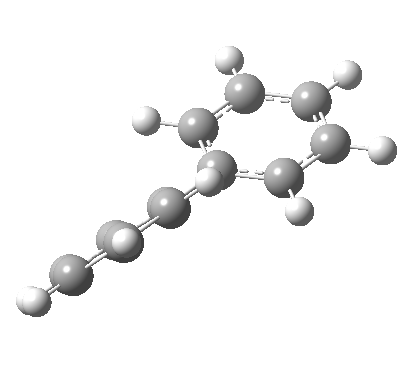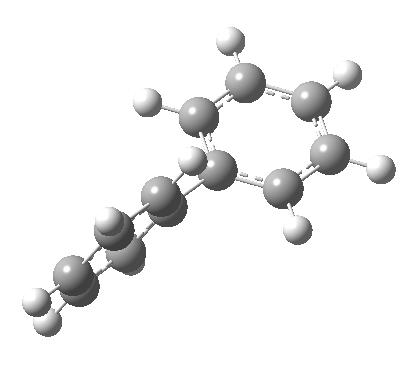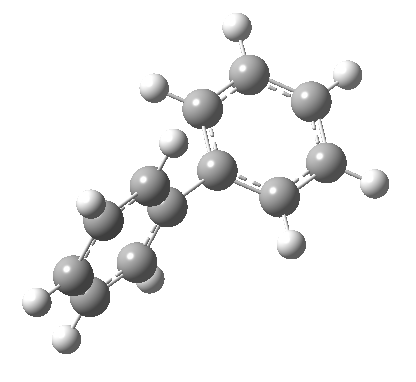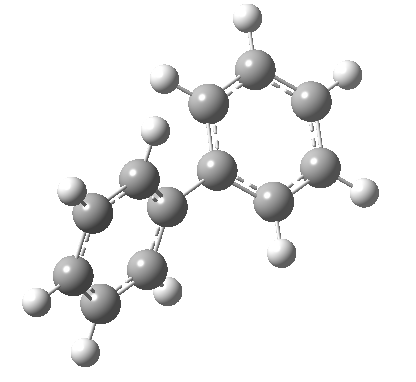
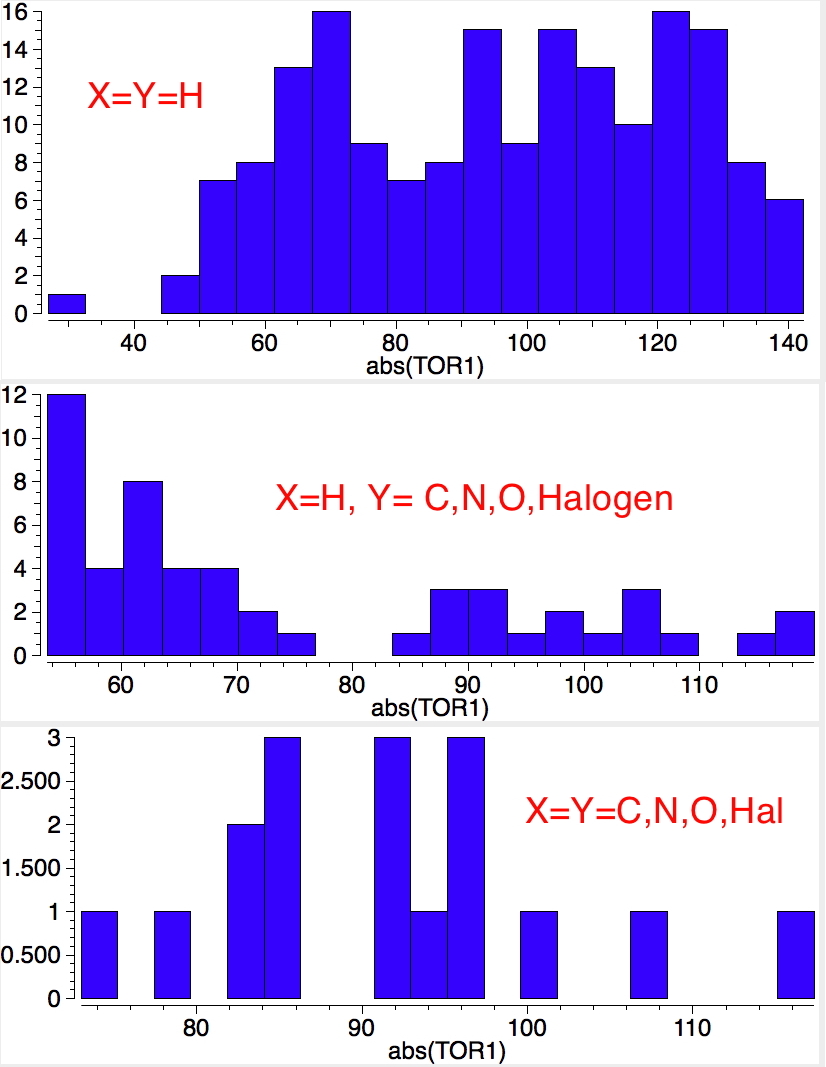
An energy profile for rotating 360° about the central (red) bond for biphenyl (X=Y=H) reveals two conformational minima which are in fact enantiomers (each has D2 symmetry) separated by two different transition states. The profile reveals two opposing forces in action. In one transition state, maximising π-π conjugation by enforcing the two aryl rings to be co-planar also forces the two pairs of ortho hydrogens to be too close, thus raising the energy. In the other transition state where the two rings are orthogonal, the two pairs of ortho hydrogens are far apart and do not interact (either repulsively or attractively). But, the π-π conjugation across the rings is completely lost. In the two (enantiomeric) conformational minima in which the rings are oriented at 45°, the H...H distances are now in the attractive van der Waals region, and the 45° orientation allows a fair bit of π-π conjugation. This accounts for why we now have a minimum rather than a transition state (a win/win).
| Transition state @0° ≡ @180° Cause: H...H clash |
Transition state @90° ≡ @270° Cause: loss of π-conjugation |
||||
 |
 |
||||
 |
|||||
| Minimum @40° ≡ @140° ≡ @220° ≡ @320° | |||||
|
|
|||||
| Enantiomerisation IRC via 0°/180° | Enantiomerisation IRC via 90° | ||||
 |
 |
||||
 The small barrier to rotation (~ 2.5-3.0 kcal/mol) means that at room temperatures rotation about the central bond will occur rapidly,
thus allowing enantiomerisation of the two minima to occur. If however, as the groups X and Y are increased in size,
a quite different rotational energy profile emerges.
The small barrier to rotation (~ 2.5-3.0 kcal/mol) means that at room temperatures rotation about the central bond will occur rapidly,
thus allowing enantiomerisation of the two minima to occur. If however, as the groups X and Y are increased in size,
a quite different rotational energy profile emerges.
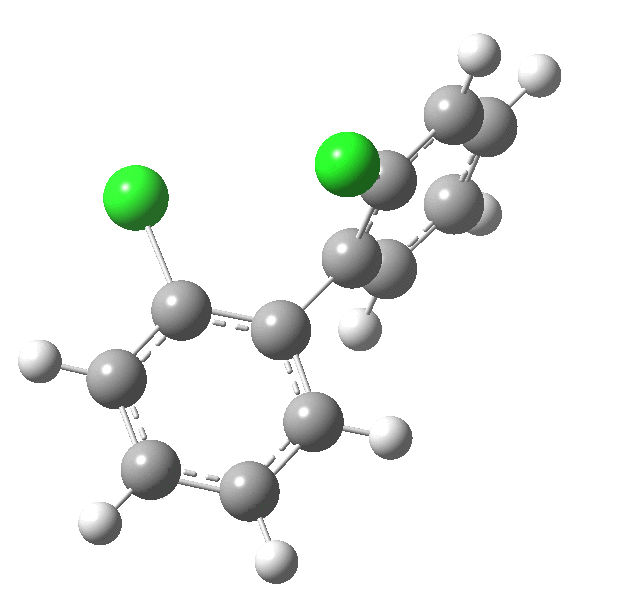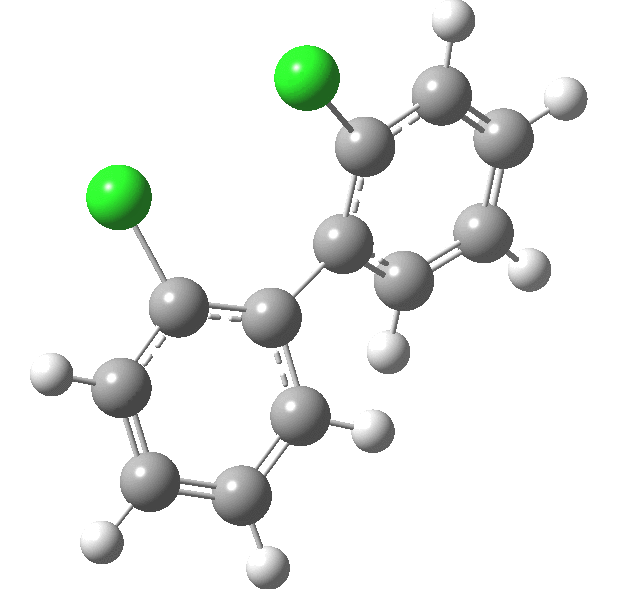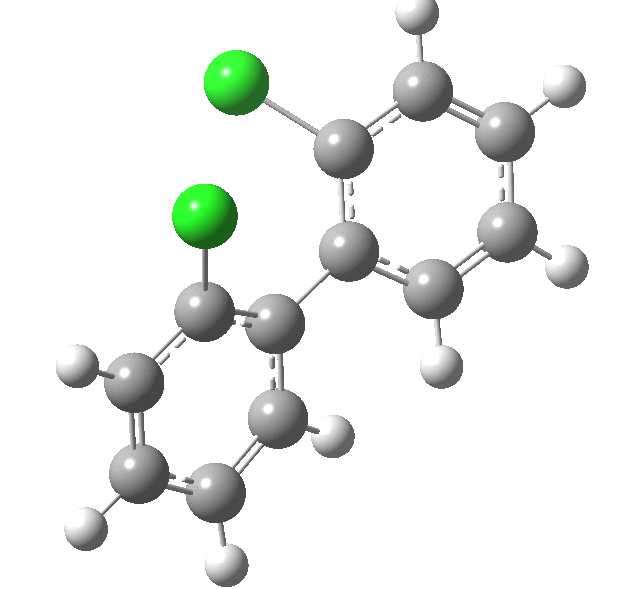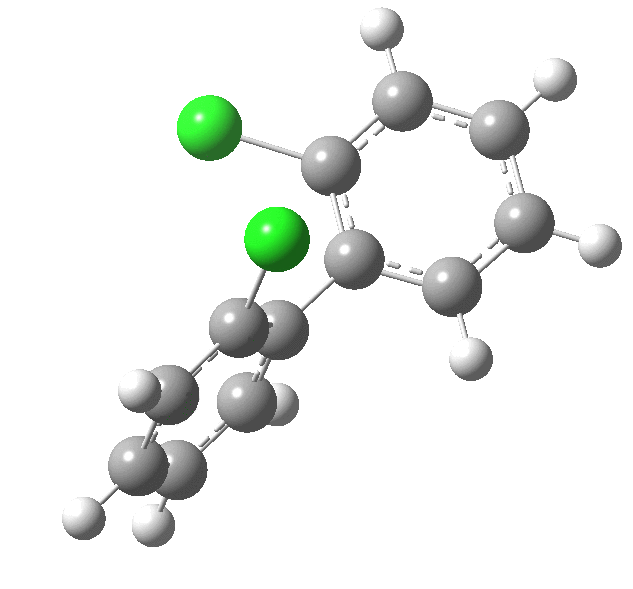
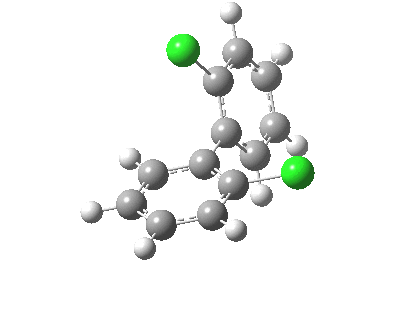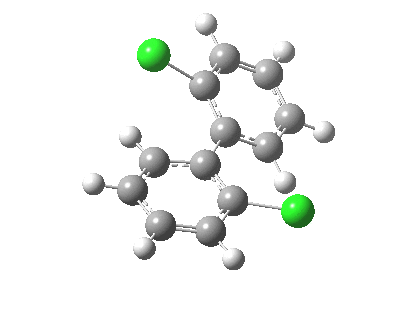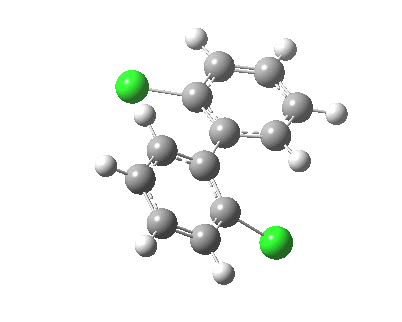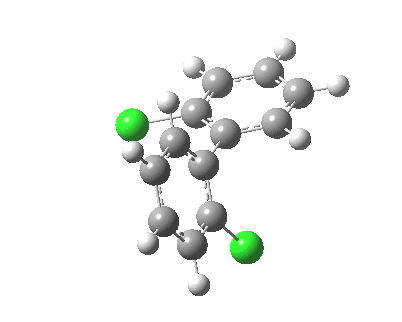
| Rotation of 2,2'-dichlorobiphenyl‡ (X=H, Y=Cl) | |
|---|---|
| High energy route: IRC via 180° Cause: Cl...Cl clash |
Low energy route: IRC via 0°/360° Cause: H...Cl clash |
 |
 |
| ‡ The artefacts are due to an imperfect reaction coordinate | |
Two transition states occur when the aryl rings are co-planar, with close approaches of H...Cl in one, or Cl...Cl in the (higher energy) other. The 45° minimum has vanished, and only two (again enantiomeric) 90° minima remain. Passing through the (lower energy) transition state requires ~20 kcal/mol for racemisation to occur. By making X and Y even larger (I,CO2H, etc) thermal racemisation can be completely suppressed and these compounds can be prepared as pure enantiomers by suitable resolution. With binaphthyl, the barriers are even larger, and a wide variety of such compounds have been prepared, resolved and in many cases adapted for use in asymmetric catalysis.
The next example tries to join up some of the types of compound discussed in this course, in illustrating atropisomerism in aryl amides. The individual enantiomers of such atropisomers are can be recognised by certain enzymes!
Several of the themes discussed in this course are reflected in how this nano-car drives!
The two preceeding examples also hint at a very important area of conformational analysis related to systems with helices (the trans-cyclo-octene can be regarded as a very simple helix). The best known example is DNA, and this is a vast area of conformational analysis we have no further time to go into!
© Henry S. Rzepa, 2010-2014. Hide|show Toolbar.