| [Related articles/posters: 077 067 095 ] |
Introduction
In our previous papers [1,2] we have reported that some nitrogen containing
heterocycles with a long alkyl chain in the molecule exhibit remarkable
antimicrobial efficiency, especially against gram-positive bacteria. In
this respect, we have also prepared some fused heterocycles [3ö5]. This
work is an extension of our study on imidazo[2,1-b]thiazolidin-3-ones bearing
an alkyl at C-2 position [5].
Results and Discussion
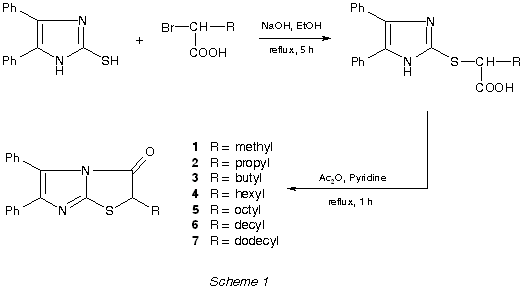
The title compounds were prepared (in about 50% yields) by intramolecular
cyclization of corresponding 2-[2-(4,5-diphenylimidazolyl)thio]alkanoic
acids (Scheme 1) using acetic anhydride and pyridine. The cyclic structures
were confirmed on the basis of 1H
and 13C NMR spectra as well as mass spectral
data (intensive peaks corresponding to the molecular ions were observed).
The results of antimicrobial activity testing revealed that some of
the prepared compounds 1÷7 exhibit moderate activity against
selected gram-positive bacteria (Table 1). Similarly to our previous findings
[1,2], the best efficiency (expressed by the integer values of minimum
inhibitory concentration - MIC) was exhibited by those derivatives where
the alkyl chain R represented hexyl or octyl. Derivatives with shorter
or longer alkyl chains showed considerably lower activity.
Table 1. Antimicrobial activity (MIC, mg.cm-3
) of the prepared imidazo[2,1-b]thiazolidin-3-ones
| Compound | Staphyloc.
aureus |
Bacillus
subtilis |
Streptococcus
faecalis |
Escherichia
coli |
Pseudomonas
aeruginosa |
Salmonella
typhimurium |
| 1 | 1000 | 1000 | >1000 | >1000 | 1000 | 1000 |
| 2 | 1000 | 1000 | 1000 | >1000 | 1000 | 1000 |
| 3 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| 4 | 10 | <10 | 100 | 1000 | <1000 | <1000 |
| 5 | 10 | 10 | 100 | 1000 | <1000 | <1000 |
| 6 | 100 | 100 | >100 | 1000 | 1000 | 1000 |
| 7 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
Experimental
General procedure for the preparation of compounds 1÷7:
A mixture of 2-[2-(4,5-diphenylimidazolyl)thio]alkanoic acid (5 mmol),
acetic anhydride (2 ml) and pyridine (5 ml) was heated under reflux for
1 h. After cooling, methanol (5 ml) was carefully added and the solvents
were evaporated under reduced pressure. Cold water (25 ml) was added to
the residue and the product was filtered and recrystallized from ethanol
to give the title compound. Yield: ~ 50%.
MIC determination:
MIC was determined by using the suspension method on solid cultivation
media [1]. As a standard for MIC value determination we have used [1-(ethoxycarbonyl)-pentadecyl]-trimethylammonium
bromide (Septonex), a commercial antiseptic agent.
Acknowledgment
Financial support of this research by the Scientific Grant Agency (VEGA,
Slovak Academy of Sciences and Ministry of Education, Bratislava, Project
No. 4144) is gratefully appreciated.
References
1. Koos, M.; Steiner, B.; Repas, M. Chem. Papers 1991,
45, 279.
2. Koos, M.; Zvakova, A.; Steiner, B.; Matulova, M. Chem. Papers
1992, 46, 50.
3. Steiner, B.; Koos, M.; Matulova, M.; Proksa, B. Monatsh. Chem.
1993, 124, 425.
4. Koos, M. Chem. Papers 1994, 48, 108.
5. Koos, M. Monatsh. Chem. 1994, 125, 1011.