| [Related articles/posters: 077 085 044 ] |
Now we wish to report the results of our study of reaction of a,b-unsaturated nitriles with sodium salt of dibenzylphosphinous acid. A number of terpene-based a,b -unsaturated nitriles (6, 10, 12, 14) having variety of carbon skeletons and different additional functions as well as the simplest unsaturated nitriles (17, 18, 19) were studied (Scheme 2), all of them, except for acrylonitrile and cynnamonitrile, were transformed to the corresponding aminophospholenes in good yields.
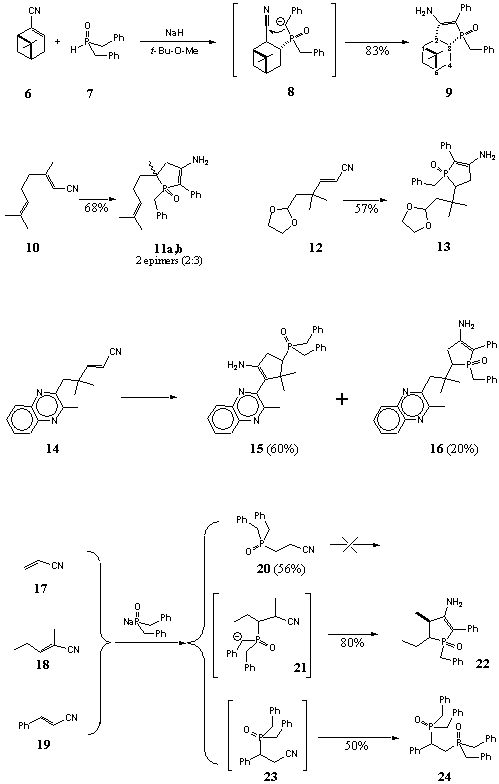
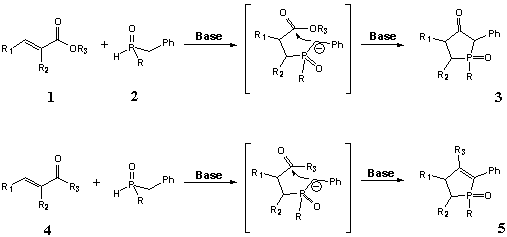
Mechanism of addition of disubstituted phosphinous acids to unsaturated esters [6] includes the primary addition of phosphinites to carbon-carbon double bond followed by addition of C-anion of benzylic type to carbonyl group (Scheme 1). Mechanism of the reaction of a,b-unsaturated nitriles seems to be the same, and addition of sodium salt of dibenzylphosphine oxide (7) to carbon-carbon double bond results in the primary adduct 8 (Scheme 2) which then undergoes intramolecular cyclization to form aminophospholene oxide 9.
1H, 13C, and 31P NMR data for compound 9 show it to be a single stereoisomer. Addition of 7 to nitrile 6 is possible only from the least hindered a -side of the molecule. Cis-fusion of the phospholene ring and pinane moiety is more preferable according to PM3 calculation rather then trans-fusion (DDH‘f = 12-15 kcal/mol). In case of the trans-fusion there must be large vicinal couplings P-C3-C2-C1 and P-C3-C4-C5, but experimental values are only 0-3 Hz that lands support to the cis-fusion. Unfortunately, neither experimental data, nor calculations allowed us to determine the configuration of the phosphorus atom.
Reaction of geranyl nitrile 10 resulted in a mixture of epimers 11a and 11b (3:2). The major isomer was isolated by crystallization, but we failed to assign its configuration.
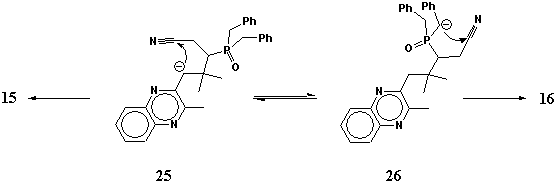
Quinoxaline-type a,b-unsaturated nitrile 14 also gave a mixture of two products, but they were structural isomers 15 and 16. The primary product of the addition of the phosphorous reagent to nitrile 14 have two types of benzyl-type methylene groups and both of them are able to take part in the cyclization (Scheme 3). Due to quite different properties of isomers 15 and 16 they were easily separated. Aminophospholene 16 was isolated as a single stereoisomer, another epimer was not detected by NMR in visible amounts even in the crude product.
Conjugated system of the unsaturated nitrile 12 is identical to that of nitrile 14and the reaction of nitrile 12 results in compound 13. As in the other cases described above, aminophospholene 13 is formed as a single stereoisomer.
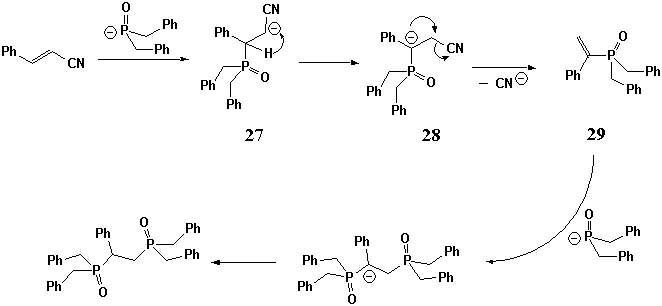
Addition of dibenzylphosphine oxide (7) to the simplest unsaturated nitriles 17, 18 and 19 proceeds also very easily, but the behavior of the primary adducts under the reaction conditions is not the same. In case of the reaction of acrylonitrile (17), primary adduct 20 demonstrates no trend to cyclization and can be isolated in good yield. The reaction of nitrile 18 resulted in formation of aminophospholene oxide 22 in good yield, while the reaction of cynnamonitrile (19) gave diphosphinoyl product 24 which is formally regarded as a product of substitution of the cyano-group in the intermediate monophosphorous adduct 23 by dibenzylphosphineoxide moiety. According to the literature, a direct substitution of cyano group by dibenzylphosphinite anion is hardly possible. Moreover, under the specified reaction conditions addition of the phosphorous reagent to cyano group is more likely [7, 8]. The following reaction sequence (Scheme 4) could explain the formation of diphosphorous compound 24. The key steps are (a) hydrogen shift in the primary adduct 27, (b) fragmentation of anion 28 by elimination of CN- and (c) addition ofΚ -P(O)(CH2Ph)2 to the substituted styrene 29 which is phosphorous analog of the corresponding a,b-unsaturated ketone.
There are four possible stereisomers of aminophospholene 22, all of them are shown in Table 1 as well as certain calculated data and experimental spin-spin couplings. Relative configuration of the methyl and phosphoryl groups was determined by NMR parameters. Dependence of the spin-spin couplings H-C-C-P and C-C-C-P on dihedral angles is known and is described by Karplus-type equation [9]. Value of the vicinal coupling H3-C3-C4-P is rather small and indicates practically perpendicular arrangement of the bonds H3-C3 and C4-P. According to the semi-empirical calculations (PM3), cis-configuration of the methyl relative to the phosphoryl oxygen (isomers 22c and 22d) just provides the value of dihedral angle H3-C3-C4-P of about 90-100‘ independently on the orientation of the ethyl group. Isomers 22c and 22d can not be distinguished by the NMR parameters listed in the Table 1. Calculated values of the dihedral angles H3-C3-C4-H4 and C5-C3-C4-P lead to very similar values of the corresponding coupling constants for both isomers.
Table 1. Some experimental and calculated parameters for the final aminophospholene oxide 22.
Resulting stereoisomers of aminophospholene 22 |
|
|
|
|
|
| Κ | 22a |
22b |
22c |
22d |
|
| Κ | Calculated data (PM3) |
||||
Experimental couplings (Hz) |
D H‘ f (kcal/mol) | -4.6 |
-2.8 |
-4.3 |
-2.6 |
JH3-C3-C4-H4 = 5.3 |
j (H3-C3-C4-H4) | 103‘ | -37‘ | -149‘ | -23‘ |
JH3-C3-C4-P = 2.2 |
j (H3-C3-C4-P) | -140‘ | -147‘ | 100‘ | 92‘ |
JC5-C3-C4-P = 8.7 |
j (C5-C3-C4-P) | 100‘ | 93‘ | -141‘ | -149‘ |
Thus, reaction of dibenzylphosphine oxide with
a,b-unsaturated nitriles in the presence of two equivalents of sodium hydride results in the formation of substituted aminophospholene oxides in good yields. Bulky substituents at the a- and g- carbons of the unsaturated nitriles cause high stereoselectivity of the cyclisation of the primary addition product and formation of the sole stereoisomer of aminophospholene oxides.1.ΚΚΚΚ Quin, L. D.; Gratz, J. P.; Barket, T. P. J. Org. Chem., 1968, 33, 1034 - 1041.
2.ΚΚΚΚ Markl, G. Angew. Chem. Internat. Edit., 1965, 4, 1023 - 1038.
3.ΚΚΚΚ Quin, L. D. The Heterocyclic Chemistry of Phosphorus: systems based on the phosphorus-carbon bond; Wiley-Interscience: New York, 1981; pp. 235 -271.
4.ΚΚΚ Bodalski, R.; Pietrusiewicz, K. M. Tetrahedron Lett., 1972, 41, 4209 - 4212.
5.ΚΚΚ Bodalski, R.; Pietrusiewicz, K. M.; Koszuk, J. Tetrahedron, 1975, 31, 1907 - 1910.
6.ΚΚΚ Purdum, W. R.; Berlin, K. D. J. Org. Chem., 1974, 39, 2904 - 2911.
7.ΚΚΚ Kirby, A. J.; Warren, S. G. The organic chemistry of Phosphorus; Elsevier: Amsterdam, 1967, pp. 39 - 168.
8.ΚΚΚΚ Edmudson, R. S. Chemical properties and reactions of phosphine chalcogenides. In The chemisry of organophosphorus compounds; Hartley, F. R. Ed.; JOHN WILEY & SONS: Chichester, 1992, Vol. 2, pp 288 - 405.
9.ΚΚΚ Gorenstein, D. G. Non-biological aspects of phosphorus-31 NMR spectroscopy. In Progress in Nuclear Magnetic Resonance Spectroscopy. 1983, Vol. 16, part 1.