| [Related articles/posters: 079 110 074 ] |

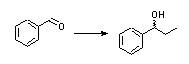 |
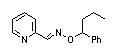 |
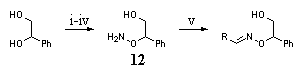 |
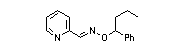 |
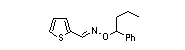 |
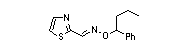 |
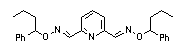 |
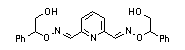 |
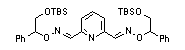 |
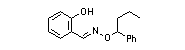 |
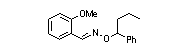 |
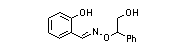 |
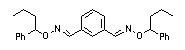 |
|
|
|
|
|
|
|
|
|
(R)-1 | 5 | 0-rt | 50 | 2 |
|
|
(R)-1 | 20 | 0-rt | 98 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(S)-3 | 20 | 0-rt | 75 | 3 |
|
|
|
|
|
|
|
|
|
(S,S)-5 | 20 | 0-rt | 25 | <1 |
|
|
(S,S)-6 | 20 | 0-rt | 51 | 17 |
|
|
(R)-7 | 5 | 0-rt | 15 | 21 |
|
|
(R)-7 | 20 | 0-rt | 100 | 30 |
|
|
(R)-7 | 20 | -78-rt | 26 |
|
|
|
|
|
|
|
|
|
|
(R)-9 | 5 | 0-rt | 4 | 17 |
| 12 | (R,R)-10 | 20 | 0-rt | 11 | 2 |