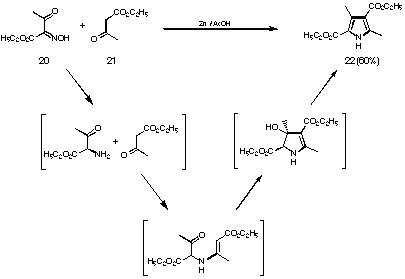
Figure 14 Mechanism for the Knorr pyrrole synthesis

Figure 14 Mechanism for the Knorr pyrrole synthesis
In order to study the feasibility of such a process a series of model studies were undertaken. It was decided to study the reaction conditions using as models the following compounds: amino acetone, amino butanone and levulinic acid. To be able to use the amino ketones for these studies we had to suitably protect the amino group. We chose the acetamino group mainly because this group is easy to synthesise and sufficiently stable even under relatively harsh reaction conditions
The group of Zav'yalov 63-66 had already shown that acetamino acetone (23) could be transformed into the pyrrole 24. The Russian group had published their results under the attractive title "Simplest chemical model of PBG synthesis". Unfortunately, no further studies in this direction have been published by the Russian chemists. Our first results using optimised conditions showed that the yield of the N-acetylpyrrole 24 could even be improved to obtain an 79% isolated yield (see Figure 15).
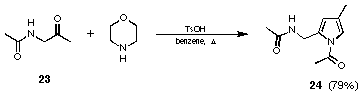
Figure 15 Synthesis of N-acetylpyrrole 24 according to Zav'yalov
We assumed that the less substituted enamine formed from acetamino acetone (23) and morpholine is an intermediate which reacts with the ketofunction of another acetamino acetone (23). Treating acetamido acetone (23) with the enamine formed from cyclohexanone and morpholine in the presence of p-toluenesulfonic acid in benzene gave the pyrrole 24 in an excellent yield of 97%. Using the same starting material with xylene as solvent and in the absence of the acid catalyst a 52% yield of the isomeric pyrrole 25 was obtained (see Figure 16). For the synthesis of the pyrrole 24 reaction conditions are used where a Broensted acid is present as catalyst. This allows one to run the reaction under milder conditions (benzene reflux compared to xylene reflux).
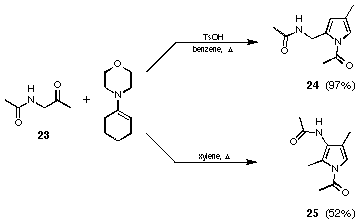
Figure 16 Selective synthesis of the pyrroles 24 and 25
Using the initial reaction conditions but changing the starting material to 1-acetaminobutan-2-one (26) unfortunately gave much less encouraging results (see Figure 17). In order to get the reaction to go we had to heat to much higher temperatures (xylene reflux). Under these conditions the mixture of the two possible regioisomers 27 and 28 in a ratio of almost 1 : 1 were obtained. The yields of the two regioisomeric pyrroles 27 and 28 were very low (4 and 5% respectively).
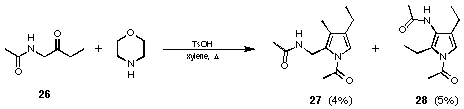
Figure 17 Formation of the pyrroles 27 and 28
The lack of reactivity and of selectivity in the reaction starting with the butanone 26 might be due to the well-known difference in reactivity between unsubstituted and substituted enamines.
We synthesised the mixture of the two regioisomeric enamines 30 and 31 in 55% yield using titanium tetrachloride as catalyst (see Figure 18). Trying to synthesise the enamines in the presence of p-toluene sulfonic acid as catalyst, higher temperatures were needed, and we directly obtained the condensation product 32 between two levulinic acid esters in 85% yield.
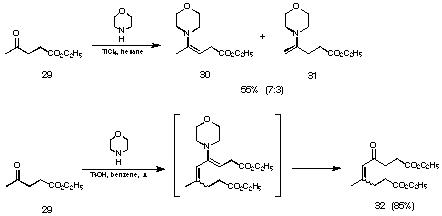
Figure 18 Synthesis of the enamines 30 and 31
Reaction bromine at -78 oC with the 7:3 mixture of enamines we obtained a distribution of the regioisomeric products which corresponds nicely to the ratio of the regioisomeric starting material (see Figure 19). For the acylation of the mixture of enamines with benzoyl chloride, we had to go to room temperature. This reaction leads to a product mixture 35 and 36, where both regioisomers can be isolated, but the minor less substituted enamine has reacted preferentially (see Figure 19).
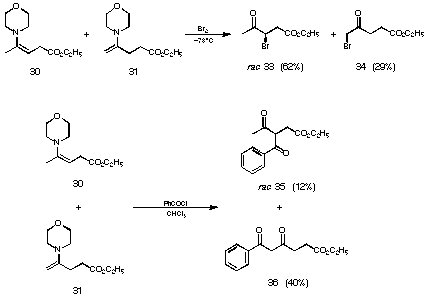
Figure 19 Reactions of the mixture of the enamines 30 and 31
Reaction of the same mixture of enamines 30 and 31 with the methyl ester of 5-acetaminolevulinic acid (37) leads to the condensation product between two levulinic acids 32 in 71% yield and only 5% yield of the N-acetyl pyrrole 38 were obtained (see Figure 20). Both reactions have to pass via the less substituted enamine.
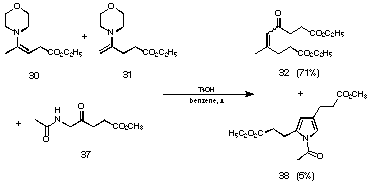
Figure 20 Reaction of the mixture of enamines 30 and 31 with the methyl ester of 5-acetaminolevulinic acid (37)
We concluded that, the reactivity of the enamines formed either from 5-acetaminolevulinic acid or from levulinic acid are not high enough. In order to form the crucial carbon-carbon bond relatively harsh reaction conditions have to be applied (higher temperatures and presence of Broensted acids). Under these conditions the equilibration between the two possible regioisomers is fast compared to the carbon-carbon bond formation. We decided to study synthetic equivalents of the enamine which hopefully should be stable under the reaction conditions.