
Studies on cyclic carbodiimides. X-ray crystal structure of
[a,c]bis{dibenzo[d,f][1,3]-diazepino}-1,3-diazetidine
aDepartamento de Química Orgánica, Facultad de Química, Universidad de Murcia, Campus de Espinardo, 30071 Murcia, Spain
bDepartamento de Cristalografía Instituto de Química-Física "Rocasolano", CSIC, Serrano 119, 28006-Madrid, Spain

Introduction
Continuing with our study on the structure, chemistry and racemization mechanisms of cyclic carbodiimides1, we started a program aimed to
the preparation of the strained chiral (S)-1,1'-binaphthyl-2,2'-diylcarbodiimide 1 and, eventually, its dimer bis[(S)-1,1'-binaphthyl-2,2'-diyl] bis(carbodiimide) 2.
With this objective point in mind we decided to try first the synthesis of the structurally related 2,2'-biphenylenecarbodiimide 3 and bis(2,2'-biphenylene)bis(carbodiimide) 4.
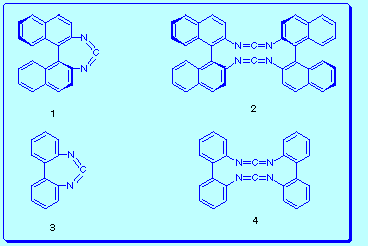
Although the results obtained in the course of our investigation have been unsuccessful in this sense (i. e. the synthetic aspect), we were stimulated to delve deeper into the mechanistic aspects of the [2+2] cycloaddition reactions of bis(carbodiimides), and in three-dimensional structure of the products obtained.
Results
In order to prepare 3 we performed two reactions, both involving as starting materials 2,2'-bis(triphenylphosphoranylideneamino)biphenyl 5 and CO2 (or Boc2O as CO2 source1):
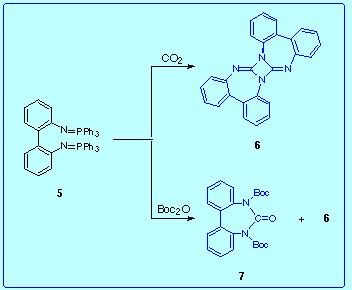 Here we show several mechanisms capable of rationalizing the results of both reactions.
Here we show several mechanisms capable of rationalizing the results of both reactions.
Taking into account the results above we hoped to prove that if the bis(carbodiimide) 4 was formed in the course of those reactions, it could undergo intramolecular [2+2] cycloaddition to [a,c]bis{dibenzo[d,f][1,3]-diazepino}-1,3-diazetidine 6. With this aim we studied the following two reactions:
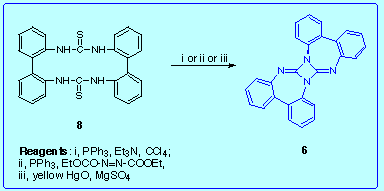
 Double dehydrosulfurization of the bis(thiourea) 8: none of the reagents we tried proved to be efficient enough, but in all the cases we obtained the diazetidine
6, albeit in low yield (3-5 %).
Double dehydrosulfurization of the bis(thiourea) 8: none of the reagents we tried proved to be efficient enough, but in all the cases we obtained the diazetidine
6, albeit in low yield (3-5 %).
 Aza-Wittig reaction between the bis(iminophosphorane) 5 and 2,2'-biphenylene bis(isothiocyanate) 9:
this reaction gave rise to a complex mixture of compounds from which we were able to isolate the diazetidine 6 in low yield (4 %).
Aza-Wittig reaction between the bis(iminophosphorane) 5 and 2,2'-biphenylene bis(isothiocyanate) 9:
this reaction gave rise to a complex mixture of compounds from which we were able to isolate the diazetidine 6 in low yield (4 %).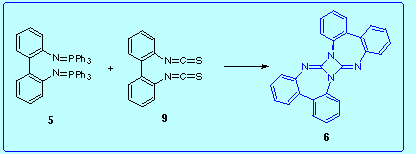
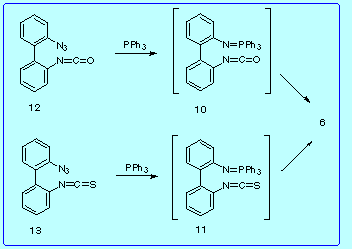
Then, it appeared interesting to know if some of the proposed intermediates, specifically 10 and 11, could be synthetized and what evolution they would experience. Toward this goal we prepared the corresponding bis(azides) 12 and 13, and examined their reactions with triphenylphosphine,
leading both to complex mixtures from which only the diazetidine 6 was isolated, again in low yield (4-5%).
At this point, we considered it necessary to investigate the reactivity of the 2,2'-biphenylene bis(carbodiimides) 14, whose structure is expected to have a greater conformational freedom than 4. Thus, we attempted to synthesize these compounds by the reaction of the bis(iminophosphorane) 5 with two types of heterocumulenes:
 With arylisocyanates:
With arylisocyanates:
This reaction led to the formation of N,N'-diarylcarbodiimides and
3-aryl-4-arylimino-dibenzo[d,f]-1,3-diazetidino[1,2-a]diazepines 15 in good yield:
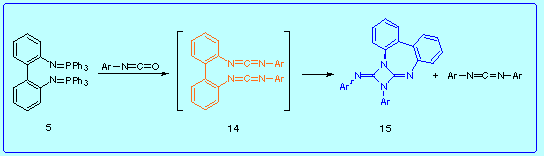
Here we propose a mechanisms to explain this result.
 With arylisothiocyanates:
With arylisothiocyanates:
Unexpecteldy, besides unreacted starting materials in this reaction we isolated N,N'-diarylcarbodiimide and the diazetidine 6. One explanation for the isolation of 6 could be the conversion of 5 to 2,2'-biphenylene bis(isothiocyanate) 9 by an abnormal aza-Wittig mechanism. Then, the combination of 5 and 9 would lead to 6 as shown above.
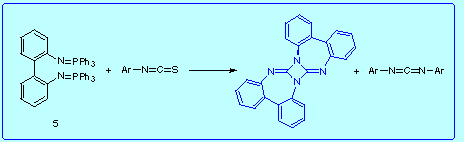 See here for mechanistic considerations.
See here for mechanistic considerations.
The reaction between 2,2'-biphenylene bis(isothiocyanate) with aryliminophosphoranes also led to diazetidines 15 in good yield together with the corresponding N,N'-diarylcarbodiimide.
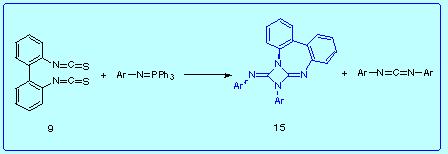 If the formation of 15 involves an intramolecular [2+2] cycloaddition of the corresponding 2,2'-biphenylene bis(carbodiimide) 14, then we can deduce that the combination of the bis(iminophosphorane) 5 and arylisothiocyanate has a greater tendency to manoeuvre like an abnormal aza-Wittig reaction than the mixture of 2,2'-biphenylene bis(isothiocyanate) with aryliminophosphoranes, since in the first reaction diazetidine 15 was not obtained.
If the formation of 15 involves an intramolecular [2+2] cycloaddition of the corresponding 2,2'-biphenylene bis(carbodiimide) 14, then we can deduce that the combination of the bis(iminophosphorane) 5 and arylisothiocyanate has a greater tendency to manoeuvre like an abnormal aza-Wittig reaction than the mixture of 2,2'-biphenylene bis(isothiocyanate) with aryliminophosphoranes, since in the first reaction diazetidine 15 was not obtained.
Discussion
 While it is intuitively reasonable that the intramolecular aza-Wittig
reaction of intermediates of the type 10 and 11 may occur more readily than the intermolecular version, it is convenient to consider that the first proposition would lead to a carbodiimide (3) with predictable strong annular strain.
While it is intuitively reasonable that the intramolecular aza-Wittig
reaction of intermediates of the type 10 and 11 may occur more readily than the intermolecular version, it is convenient to consider that the first proposition would lead to a carbodiimide (3) with predictable strong annular strain.
On the other hand, the failure in the preparation of the cyclic carbodiimide 3 can be attributed to their instability, since Wentrup2 proposed 3 as a reactive intermediate which quickly cyclodimerized to the diazetidine 6. Is this the way in which 6 was formed in our reactions
 In the light of our experience on the synthesis of cyclic bis(carbodiimides),we may expect that the bis(carbodiimide) 4 would be formed to a reasonable extent in the reaction of the bis(iminophosphorane) 5 with 2,2'-biphenylene bis(isothiocyanate). If the bis(carbodiimide) 4 cyclodimerized to diazetidine 6, why in so very low yield Is that due to the conversion itself or to the low yield in the formation of 4
In the light of our experience on the synthesis of cyclic bis(carbodiimides),we may expect that the bis(carbodiimide) 4 would be formed to a reasonable extent in the reaction of the bis(iminophosphorane) 5 with 2,2'-biphenylene bis(isothiocyanate). If the bis(carbodiimide) 4 cyclodimerized to diazetidine 6, why in so very low yield Is that due to the conversion itself or to the low yield in the formation of 4
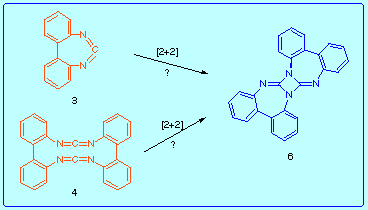
 A comment about the structure of cyclic bis(carbodiimides): due to the
chirality of cumulenes, each carbodiimide fragment exists as a mixture of R and S enantiomers, thus bis(carbodiimides) could exist as d,l or meso isomers. To clarify this problem we have represented below these possibilities in a schematic form: the three stroke fragment representing the carbodiimide (the central bold part being the N=C=N bond) and the sphere representing the bridge between such sub-units:
A comment about the structure of cyclic bis(carbodiimides): due to the
chirality of cumulenes, each carbodiimide fragment exists as a mixture of R and S enantiomers, thus bis(carbodiimides) could exist as d,l or meso isomers. To clarify this problem we have represented below these possibilities in a schematic form: the three stroke fragment representing the carbodiimide (the central bold part being the N=C=N bond) and the sphere representing the bridge between such sub-units: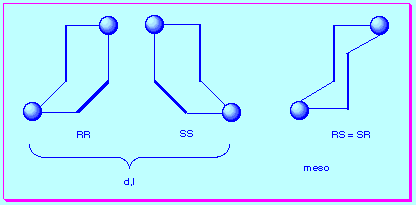 Unfortunately the configurational stability of the carbodiimides is very low (the open chain carbodiimides have racemization energy barriers around 7 kcal mol-1) (1 cal = 4.184 J)3 and that prevented the isolation of the posible isomers. An accurate molecular model of bis(carbodiimide) 4 could help us to know if there exists any steric interference for the isomerization (meso<->d,l). Furthermore, the three-dimensional structure could reveal whether the relative disposition of the carbodiimide fragments is favourable to the [2+2] cycloaddition.
Unfortunately the configurational stability of the carbodiimides is very low (the open chain carbodiimides have racemization energy barriers around 7 kcal mol-1) (1 cal = 4.184 J)3 and that prevented the isolation of the posible isomers. An accurate molecular model of bis(carbodiimide) 4 could help us to know if there exists any steric interference for the isomerization (meso<->d,l). Furthermore, the three-dimensional structure could reveal whether the relative disposition of the carbodiimide fragments is favourable to the [2+2] cycloaddition.
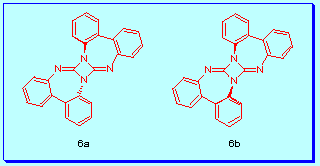
 Nevertheless, the
X-ray difraction analysis showed the diazetidine 6 as
only one of the two possible isomers( 6a and 6b),
the one with an inversion centre, 6a, taking into account
the pyramidalized nature of the diazetidine nitrogen atoms.
Nevertheless, the
X-ray difraction analysis showed the diazetidine 6 as
only one of the two possible isomers( 6a and 6b),
the one with an inversion centre, 6a, taking into account
the pyramidalized nature of the diazetidine nitrogen atoms.
Could the two isomers of 6 equilibrate in
solution
We hope that solution VT-NMR and solid state 13C NMR experiments
will give us the answer. This fact bring about an interrogation point about
the topology of the possible transition states wich leads to the diazetidine
6.
Acknowledgments
Thanks are given to Professor J. Elguero (CSIC, Madrid)
for useful comments on part of this work.
We thank the Dirección General de Investigación Científica y Técnica (DGICYT) for financial support (Project PB92-0984).



![]() Double dehydrosulfurization of the bis(thiourea) 8: none of the reagents we tried proved to be efficient enough, but in all the cases we obtained the diazetidine
6, albeit in low yield (3-5 %).
Double dehydrosulfurization of the bis(thiourea) 8: none of the reagents we tried proved to be efficient enough, but in all the cases we obtained the diazetidine
6, albeit in low yield (3-5 %).


![]() With arylisocyanates:
With arylisocyanates:


![]() In the light of our experience on the synthesis of cyclic bis(carbodiimides),we may expect that the bis(carbodiimide) 4 would be formed to a reasonable extent in the reaction of the bis(iminophosphorane) 5 with 2,2'-biphenylene bis(isothiocyanate). If the bis(carbodiimide) 4 cyclodimerized to diazetidine 6, why in so very low yield Is that due to the conversion itself or to the low yield in the formation of 4
In the light of our experience on the synthesis of cyclic bis(carbodiimides),we may expect that the bis(carbodiimide) 4 would be formed to a reasonable extent in the reaction of the bis(iminophosphorane) 5 with 2,2'-biphenylene bis(isothiocyanate). If the bis(carbodiimide) 4 cyclodimerized to diazetidine 6, why in so very low yield Is that due to the conversion itself or to the low yield in the formation of 4

![]() Nevertheless, the
X-ray difraction analysis showed the diazetidine 6 as
only one of the two possible isomers( 6a and 6b),
the one with an inversion centre, 6a, taking into account
the pyramidalized nature of the diazetidine nitrogen atoms.
Nevertheless, the
X-ray difraction analysis showed the diazetidine 6 as
only one of the two possible isomers( 6a and 6b),
the one with an inversion centre, 6a, taking into account
the pyramidalized nature of the diazetidine nitrogen atoms.
