| [Molecules: 2] [Related articles/posters: 026 009 054 061 083 ] |
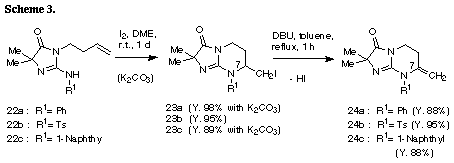
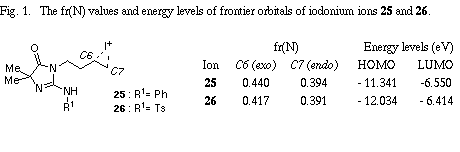
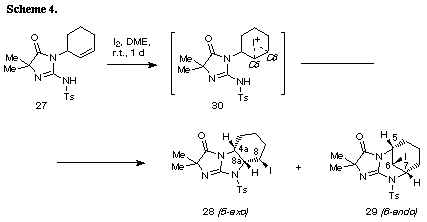
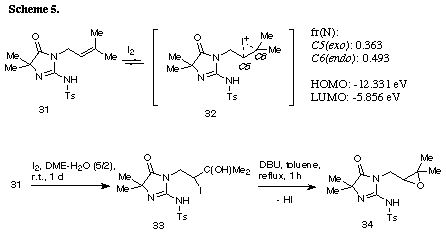
The similar reaction of 3-(3-methylbut-2-enyl) substrate 31 with iodine gave the unreacted 31 in recovery of 78%. The PM3 molecular orbital calculations of the iodonium ion 32 suggested the predominant formation of 6-endo cyclization product, although the energy difference between the frontier orbitals (dE= 6.475 eV) of the iodonium ion 32 was somewhat larger than those of 3-allyl substrate (dE= 5.720 eV)2 and 3-(but-3-enyl) substrate 26 (dE= 5.620 eV). The similar reaction of 31 in the presence of water gave iodohydrin 33 in 78% yield and the regiochemistry of the addition of hypoiodide was consistent with the PM3 calculation results. The treatment of 33 with DBU gave epoxide 34 in 75% yield. These results suggest that iodonium ion 32 is expected to form and that the successive nucleophilic attack of the amino nitrogen on the ion 32 is probably blocked because of serious steric interaction between both reaction sites.8
Noguchi, M.; Okada, H.; Watanabe, M.; Okuda, K.; Nakamura, O. Tetrahedron 1996, 52, 6581.