A short and efficient asymmetric synthesis of 5-substituted D3 pyrrolin-2-ones. A new approach to 2-pyrrolidones and pyrrolidines
Pedro MERINO*, Francisco Merchan and Tomas Tejero
Departamento de Quimica Organica, ICMA, Universidad de Zaragoza, CSIC, Zaragoza, Spain
In recent years polyhydroxylated pyrrolidines and pyrrolidones have attracted
much attention, due to their ability to act as selective glycosidase inhibitors
with a variety of therapeutic effects.[1]
Most of the synthetic strategies directed towards the construction of those
compounds involve the use of naturally occurring polyhydroxylated compounds.
Whereas there have been several reports concerning this issue,[2] very scarcely has there been reported further
de novo stereoselective construction of the pyrrolidine ring.[3]
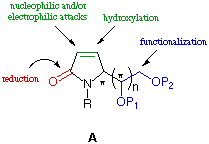
In this context C-5 substituted pyrrolin-2-ones A
can serve as templates for the construction of a number of highly
functionalized 2-pyrrolidones and pyrrolidines by exploiting (i) functionalization of the double bond, (ii) reduction of the amide group and (iii) the latent functionality of the side chain
 Earlier reports from our laboratory have established the utility of nitrones as
electrophiles in highly stereoselective additions. Those studies resulted in
the development of useful strategies for the asymmetric synthesis of
a-amino acids,[4] a-amino
nitriles,[5] a-amino aldehydes[6] and allyl amines.[7]
Earlier reports from our laboratory have established the utility of nitrones as
electrophiles in highly stereoselective additions. Those studies resulted in
the development of useful strategies for the asymmetric synthesis of
a-amino acids,[4] a-amino
nitriles,[5] a-amino aldehydes[6] and allyl amines.[7]
As shown in the Scheme we describe herein a new approach to compounds of
type A starting from nitrone 1 which is readily available in high
quantity from D-glyceraldehyde.[8]
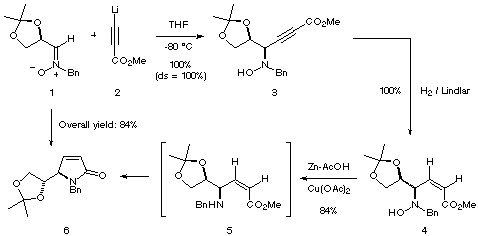
The addition of lithium propiolate 2 to nitrone 1 took place with
complete syn selectivity and quantitative yield to afford the prop-2-ynyl
hydroxylamine 3. The relative configuration of 2 was reasonably
assigned on the basis of our previous findings concerning the addition of
acetylene derivatives to the nitrone 1.[7] The observed syn
selectivity is in good agreement with the model previously proposed by us for
the addition of other nucleophiles.[9]
The selective hydrogenation of the triple bond was achieved in 1 h at ambient
temperature and atmospheric pressure using the Lindlar catalyst. The corresponding
allyl hydroxylamine 4 was obtained in quantitative yield.[10] Further deoxygenation of hydroxylamine
4 was accomplished with Zn-Cu in acetic acid as a solvent. Under these
conditions, the resulting allyl amine 5 could not be isolated since it
cyclized spontaneously to the pyrrolin-2-one 6 which was obtained in 84%
yield after purification by column chromatography.[11]
In summary, we have presented a convenient and straightforward method of preparing
enantiomerically pure d3-pyrrolin-2-ones, suitable starting
materials for the construction of polyfunctionalized pyrrolidines and
2-pyrrolidones. Interesting features of this synthesis include the high level
of diastereoselection and the ease of the cyclization step.
Application of compounds A to the synthesis of highly functionalized
pyrrolidines are currently being evaluated and will be reported in due
course.
Acknowledgement
Financial support from MEC (Project PB94-0598, DGICYT, Madrid,
Spain) is gratefully acknowledged.
References and Notes

