Research
Braddock group research can be broadly defined as stereoselective organic synthesis, and encompasses work in the area of natural product synthesis, novel methodology and asymmetric organocatalysis. A brief summary of some of our published work to date is given below.
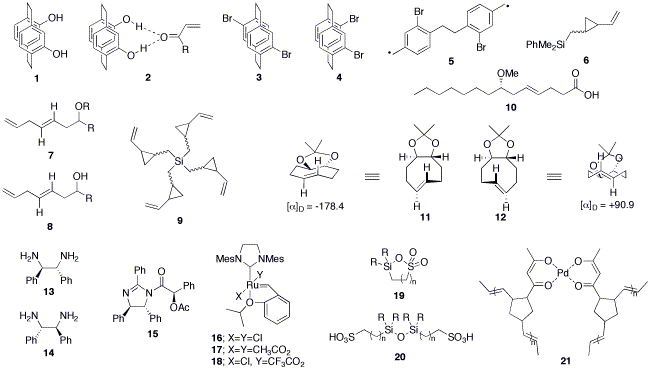
The Braddock group has developed the chemistry of symmetrically 4,12-disubstituted[2.2]paracyclophanes, exemplified by planar chiral PHANOL 1,1 which acts as an asymmetric activator of carbonyl groups by double hydrogen bonding e.g. as 2,2,3 and by the application of microwave irradiation for the isomerisation of 4,16-dibromo[2.2]paracyclophane 3 into 4,12-dibromo[2.2]paracyclophane 4 via diradical 5.4
Vinyl silylcyclopropyl reagent 6 has been developed for the "cyclopropylmethylsilyl terminated Prins reaction" with activated acetals or aldehydes to give skipped diene ethers 75 and alcohols 86 with exclusive control of the E-olefin geometry. The usefulness of tetrafunctionalised vinyl silane 9 was demonstrated for the preparation of marine natural product lygnbic acid 10, where the Prins reaction simultaneously installed the long-chain fatty acid, the methoxy group and the olefin with exclusive E-geometry.7
The first asymmetric synthesis of a pair of trans-cyclooctene isomers, locked either in a chair 11 or a twist 12 has recently been accomplished.8 A convenient gram-scale preparation of the valuable asymmetric diamine ligands 13 and 14 has been reported via the separation of N-acetylmandelates of isoamarine 15.9 Unprecedented anionic ligand exchange between Hoveyda-Grubbs ruthenium benzylidenes of the type 16 and 17 to give mixed species 18 has recently been uncovered,10 and this has led to a novel method for the immobilisation of catalysts through the 'anionic' position.11
Other work includes the in-situ use of palladium (0) ruthenium benzylidene combinations to catalyse tandem allylic isomerisation and ring closing metathesis,12,13 the synthesis of silasultones 19 via double dehydrative cyclisation of siloxanes 20,14 and the preparation of a highly-loaded palladium (II) ROMP polymer 21.15
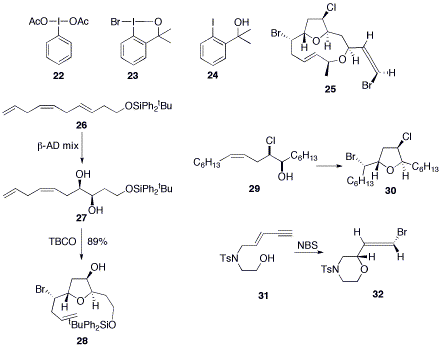
The Braddock group has also been interested in developing new methods for electrophilic bromination. A stoichiometric combination of di(acetoxy)iodobenzene 22 and lithium bromide allows for the electrophilic monobromination of electron rich aromatics and heteroaromatics.16 It was found that bromoiodinane 23 can also function as an electrophilic donor of bromine.17 A catalytic bromination reaction was then developed using carbinol 24 as the catalyst and N-bromosuccinamide (NBS) as the stoichiometric bromine source.18 Further work has identified dimethylformamide, dimethylacetamide and tetramethylguanidine as organocatalysts for the transfer of electrophilic bromine from NBS to alkenes.19
A biosynthetically-inspired asymmetric synthesis of the halogenated marine natural product family, the obtusallenes, has also been pursued. This family is exemplified by obtusallene II (25), and an internally self-consistent hypothesis for their biogenesis via multiple electrophilic bromination of a C15 fatty acid has been proposed.20 Asymmetric synthesis of of the core of obtusallene II has been achieved by an unprecedented regioselective asymmetric dihydroxylation of a trans versus cis versus terminal olefin of triene 26 to give diol 27, followed by a highly diastereoselective bromoetherification to give tetrahydrofuran 28. Alternatively, bromoetherification of halohydrin 29 gave the fully elaborated core 30 of obtusallene II.21 With relevance to the C1-C4 bromoallene fragment of obtusallene II, the stereochemical course of bromoetherification of N-tosylate-E-enyne 31 into bromoallene 32 proved to proceed with stereospecific syn addition.22
[1] Braddock, D. C.; MacGilp, I.; Perry, B. G. J. Org. Chem., 2002, 67, 8679-8681. DOI
[2] Braddock, D. C.; MacGilp, I. D.; Perry, B. G. Synlett, 2003, 1121-1124. DOI
[3] Braddock, D. C.; MacGilp, I. D.; Perry, B. G. Adv. Synth. Catal., 2004, 346, 1117-1130. DOI
[4] Braddock, D. C.; Ahmad, S. M.; Douglas, G. T. Tetrahedron Lett. 2004 45, 6583-6585. DOI
[5] Braddock, D. C.; Badine, D. M.; Gottschalk, T. Synlett 2001, 1909-1912. DOI
[6] Braddock, D. C.; Badine, T.; Gottschalk, T.; Matsuno, A.; Rodriguez-Lens, M. Synlett 2003, 345-348. DOI
[7] Braddock, D. C.; Matsuno, A. Synlett 2004, 2521-2524. DOI
[8] Braddock, D. C.; Cansell, G.; Hermitage, S. A.; White, A. J. P. Tetrahedron:Asymmetry 2004, 15, 3123-3129. DOI
[9] Braddock, D. C.; Butterworth, J.; Hermitage, S. A.; White, A. J. P. Adv. Synth. Cat. 2006, 348, 911-916. DOI
[10] Tanaka, K.; Böhm, V. P. W.; Chadwick, D.; Roeper, M.; Braddock, D. C. Organometallics 2006 25, 5696-5698. DOI
[11] Braddock, D. C.; Tanaka, K.; Chadwick, D.; Böhm, V. P. W.; Roeper, M. Tetrahedron Lett., 2007, 48, 5301-5303.DOI
[12] Braddock, D. C.; Wildsmith, A. J. Tetrahedron Lett. 2001, 42, 3239-3242. DOI
[13] Braddock, D. C.; Matsuno, A. Tetrahedron Lett. 2002, 43, 3305-3308. DOI
[14] Braddock, D. C.; Peyralans, J. J.-P. Tetrahedron 2005, 61, 7233-7240. DOI
[15] Braddock, D. C.; Chadwick, D.; Lindner-López, E. Tetrahedron Lett. 2004, 45, 9021-9024. DOI
[16] Braddock, D. C.; Cansell, G.; Hermitage, S. A. Synlett 2004, 461-464. DOI
[17] Braddock, D. C.; Cansell, G.; Hermitage, S. A.; White, A. J. P. Chem. Commun. 2006, 1442-1444. DOI
[18] Braddock, D. C.; Cansell, G.; Hermitage, S. A. Chem. Commun. 2006, 2483-2485. DOI
[19] Ahmad, S. M.; Braddock, D. C.; Cansell, G.; Hermitage, S. A. Tetrahedron Lett. 2007, 48, 915-918. DOI
[20] Braddock, D. C. Org. Lett. 2006, 8, 6055-6058. DOI
[21] Braddock, D. C.; Bhuva, R.; Millan, D. S.; Perez-Fuertes, Y.; Roberts, C. A.; Sheppard, R. N.; Solanki, S.; Stokes, E. S. E.; White, A. J. P. Org. Lett. 2007, 9, 445-448. DOI
[22] Braddock, D. C.; Bhuva, R.; Perez-Fuertes, Y.; Roberts, C. A.; Stokes, E. S. E.; White, A. J. P. Chem. Commun. 2008, 1419-1421. DOI