April 9th, 2011
Understanding why and how proteins fold continues to be a grand challenge in science. I have described how Wrinch in 1936 made a bold proposal for the mechanism, which however flew in the face of much of then known chemistry. Linus Pauling took most of the credit (and a Nobel prize) when in a famous paper[1] in 1951 he suggested a mechanism that involved (inter alia) the formation of what he termed α-helices. Jack Dunitz in 2001[2] wrote a must-read article[3] on the topic of “Pauling’s Left-handed α-helix” (it is now known to be right handed). I thought I would revisit this famous example with a calculation of my own and here I have used the ωB97XD/6-311G(d,p) DFT procedure[4] to calculate some of the energy components of a small helix comprising (ala)6 in both left and right handed form.
Read the rest of this entry »
References
- L. Pauling, R.B. Corey, and H.R. Branson, "The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain", Proceedings of the National Academy of Sciences, vol. 37, pp. 205-211, 1951. https://doi.org/10.1073/pnas.37.4.205
- J.D. Dunitz, "Pauling's Left-Handed α-Helix", Angewandte Chemie International Edition, vol. 40, pp. 4167-4173, 2001. https://doi.org/10.1002/1521-3773(20011119)40:22<4167::aid-anie4167>3.0.co;2-q
- https://doi.org/
- K.S. Thanthiriwatte, E.G. Hohenstein, L.A. Burns, and C.D. Sherrill, "Assessment of the Performance of DFT and DFT-D Methods for Describing Distance Dependence of Hydrogen-Bonded Interactions", Journal of Chemical Theory and Computation, vol. 7, pp. 88-96, 2010. https://doi.org/10.1021/ct100469b
Tags: alpha-helix, aqueous solutions, chiroptical, conformational analysis, dielectric, energy, energy components, high energy species, Historical, Jack Dunitz, Julia Contreras-Garcia, protein, solvation algorithms, Tutorial material, watoc11
Posted in Interesting chemistry | 4 Comments »
April 4th, 2011
Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λmax 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model. Andy suggested this latter was due to stacking. Here, the calculated spectrum of a stacked dimer is explored.
Read the rest of this entry »
Tags: Andy Mclean, copper phthalocyanine, energy, Historical, TD-DFT, X-ray
Posted in Interesting chemistry | No Comments »
April 4th, 2011
Most scientific theories emerge slowly, over decades, but others emerge fully formed virtually overnight as it were (think Einstein in 1905). A third category is the supernova type, burning brightly for a short while, but then vanishing (almost) without trace shortly thereafter. The structure of DNA (of which I have blogged elsewhere) belongs to the second class, whilst one of the brightest (and now entirely forgotten) examples of the supernova type concerns the structure of proteins. In 1936, it must have seemed a sure bet that the first person to come up with a successful theory of the origins of the (non-random) relatively rigid structure of proteins would inevitably win a Nobel prize. Of course this did happen for that other biologically important system, DNA, some 17 years later. Compelling structures for larger molecules providing reliable atom-atom distances based on crystallography were still in the future in 1936, and so structural theories contained a fair element of speculation and hopefully inspired guesswork (much as cosmological theories appear to have nowadays!).
Read the rest of this entry »
Tags: Cambridge, chair, Derek Barton, Dorothy Wrinch, energy, high energy species, Historical, mathematician, organic chemist, Patrick Coffey, relative free energy, thermodynamics, Tutorial material
Posted in Interesting chemistry | 2 Comments »
March 30th, 2011
I am at the ACS meeting, attending a session on chemistry and the Internet. This post was inspired by Chemicalize, a service offered by ChemAxon, which scans a post like this one, and identifies molecules named. I had previously used generic post taggers, which frankly did not work well in identifying chemical content. So this is by way of an experiment. I list below some of the substances about which I have blogged, to see how the chemicalizer works. Read the rest of this entry »
Tags: ACS, chemical content
Posted in Chemical IT | 7 Comments »
March 9th, 2011
In the previous post I pondered the colour of Monastral blue (copper phthalocyanine). Something did not quite fit, and so I speculated that perhaps some oxidation of the pigment might give a new species. This species (Cambridge code FEGJOQ) comprises two parts of copper phthalocyanine, 1 part of the corresponding cation, and 1 part of triodide anion. Looking at the packing of this system, I spotted something I had seen some time ago in NaI2.Acetone, namely an infinitely long and absolutely straight chain of iodine atoms, a molecular wire if you like.
Read the rest of this entry »
Tags: Cambridge, molecular wire, phthalocyanine
Posted in Interesting chemistry | 2 Comments »
March 8th, 2011
The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else. Fortunately, much of that story is actually recorded on film (itself a unique archive dating from 1933 and being one of the very first colour films in existence!). Patrick Linstead, a young chemist then (he eventually rose to become rector of Imperial College) tells the story himself here. It is well worth watching, if only for its innocent social commentary on the English class system (and an attitude to laboratory safety that should not be copied nowadays). Here I will comment only on its colour and its aromaticity.
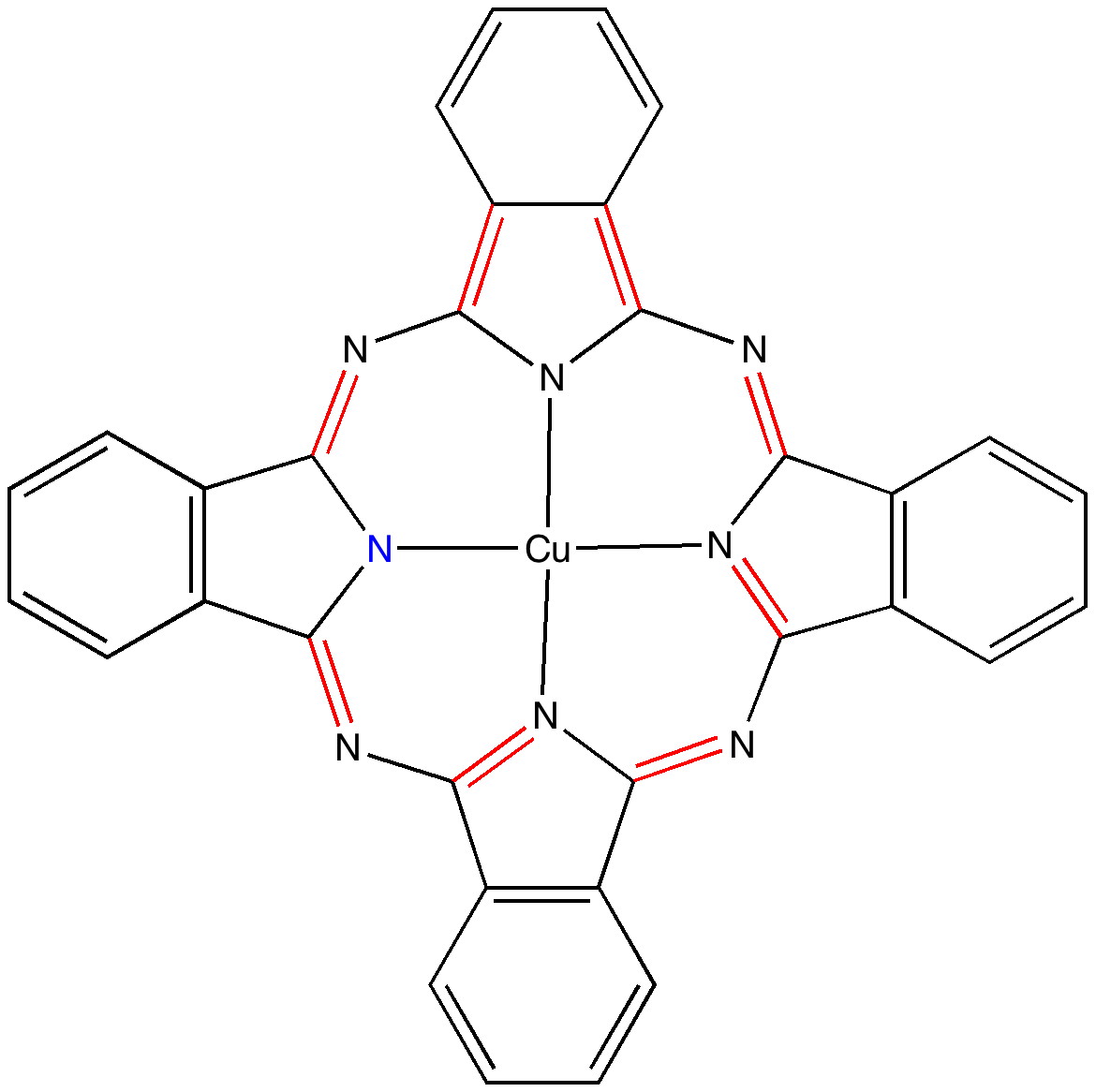
Copper phthalocyanine
Read the rest of this entry »
Tags: 18 electron aromaticity, chemical serendipity, Historical, HTML, HTML element, Imperial College, Missouri, Monastral blue, Patrick Linstead, phthalocyanine, Phthalocyanine Blue BN, Phthalocyanines, Pigments, rector, young chemist
Posted in Interesting chemistry | 6 Comments »
March 5th, 2011
In this earlier post, I noted some aspects of the calculated structures of both Z- and B-DNA duplexes. These calculations involved optimising the positions of around 250-254 atoms, for d(CGCG)2 and d(ATAT)2, an undertaking which has taken about two months of computer time! The geometries are finally optimised to the point where 2nd derivatives can be calculated, and which reveal up to 756 all-positive force constants and 6 translations and rotations which are close to zero! This now lets one compute the thermodynamic relative energies using ωB97XD/6-31G(d) (for 2nd derivatives) and 6-31G(d,p) (for dispersion terms). All geometries are optimized using a continuum solvent field (water), and are calculated, without a counterion, as hexa-anions. Read the rest of this entry »
Tags: 6-31G(d), ATAT duplex, B-DNA, CGCG duplex, dispersion energy corrections, energy, thermodynamic stability, Tutorial material, watoc11, wB97XD, Z-DNA
Posted in Interesting chemistry | 4 Comments »
March 5th, 2011
My colleague Bill Griffith has again come up with another colour challenge: that of the ancient semi-precious stone Lapis Lazuli, mined in the mountains of Afghanistan for more than 6000 years and used by painters in some medieval paintings of the Virgin, the Wilton diptych etc.
Read the rest of this entry »
Tags: Afghanistan, Bill Griffith, Lapis Lazuli, Missouri, trisulfide radical anion, Web browser
Posted in Interesting chemistry | 2 Comments »
March 1st, 2011
Much like chocolate, some of us metallaholics cannot get enough. So WUQXIP proved an irresistible frolic (DOI: 10.1021/om020789h). Let us start with benzene. It can have metals added in two ways, whilst preserving its essential aromaticity.
Read the rest of this entry »
Tags: Albrecht Salzer, Frank Podewils, Martin Kaupp, metal, metal delight, metal sandwich, metal-metal bond, metallabenzene, metallocene, three-centre bonding, triple metal bonding delight, Triple metal delight, Trixie Wagner, Ulli Englert, Ulrike Effertz
Posted in Hypervalency | No Comments »
