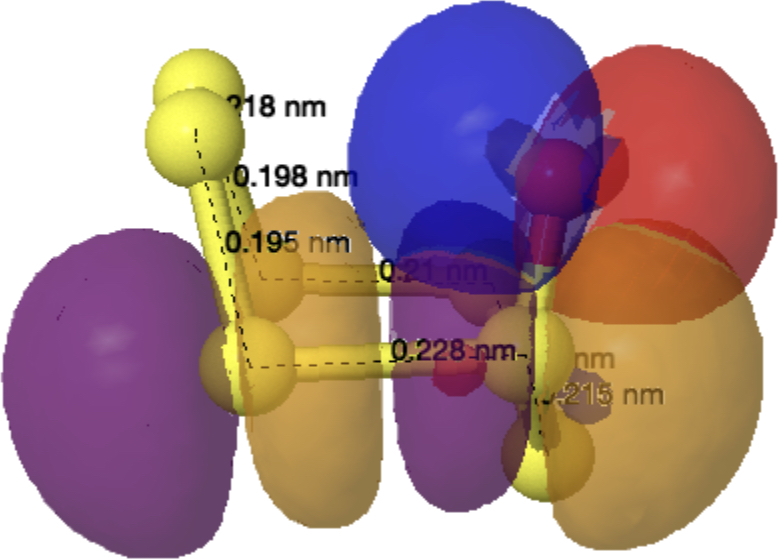
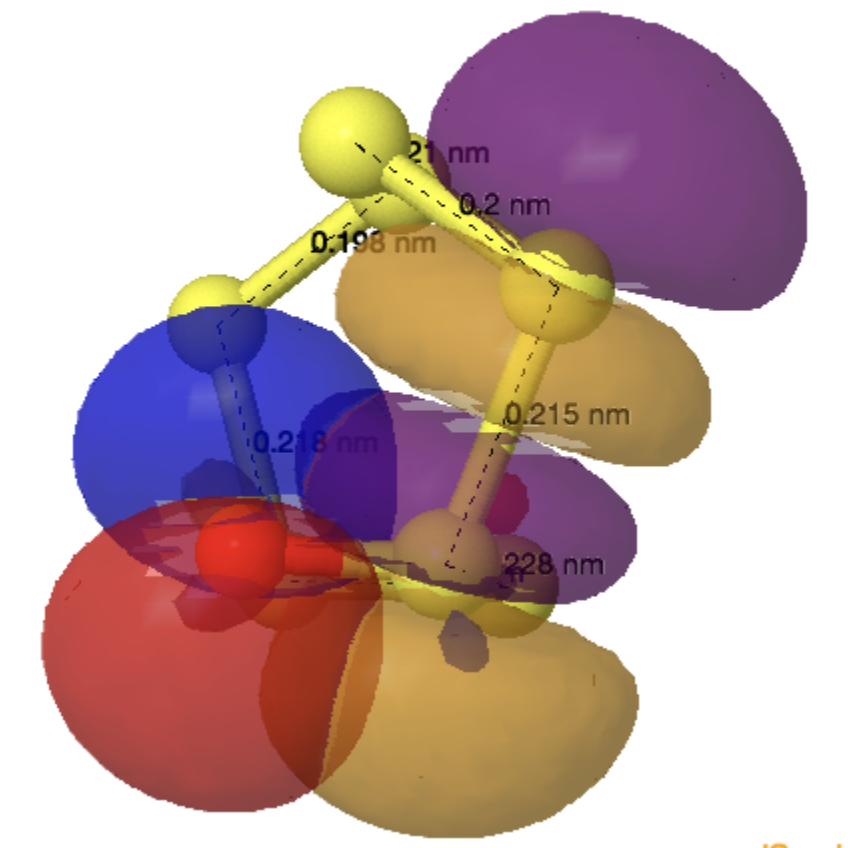
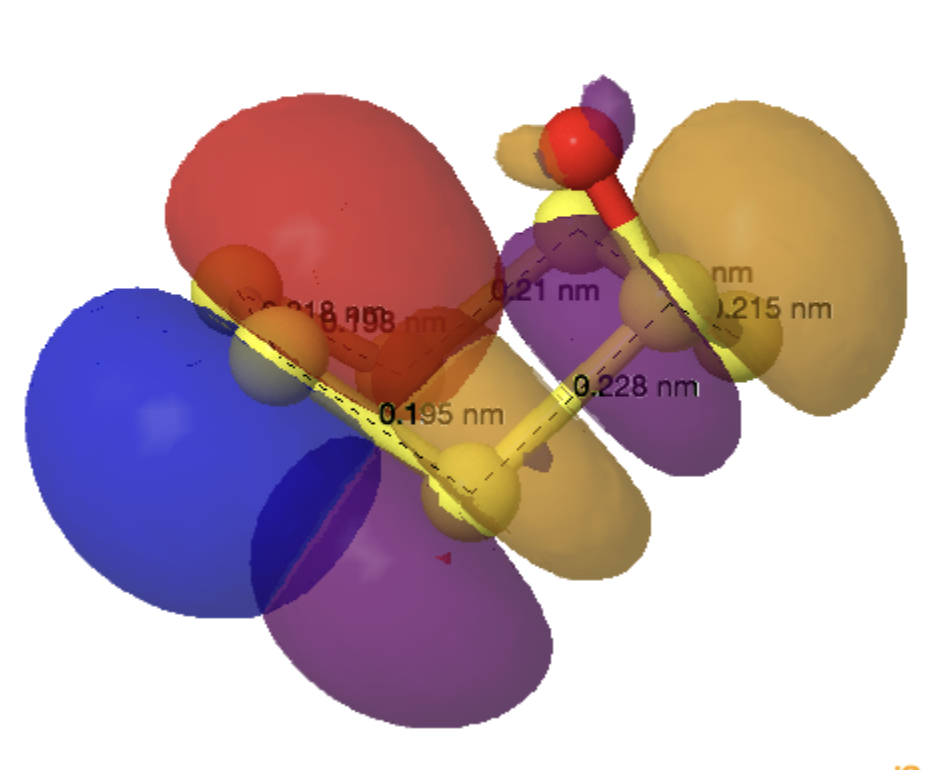
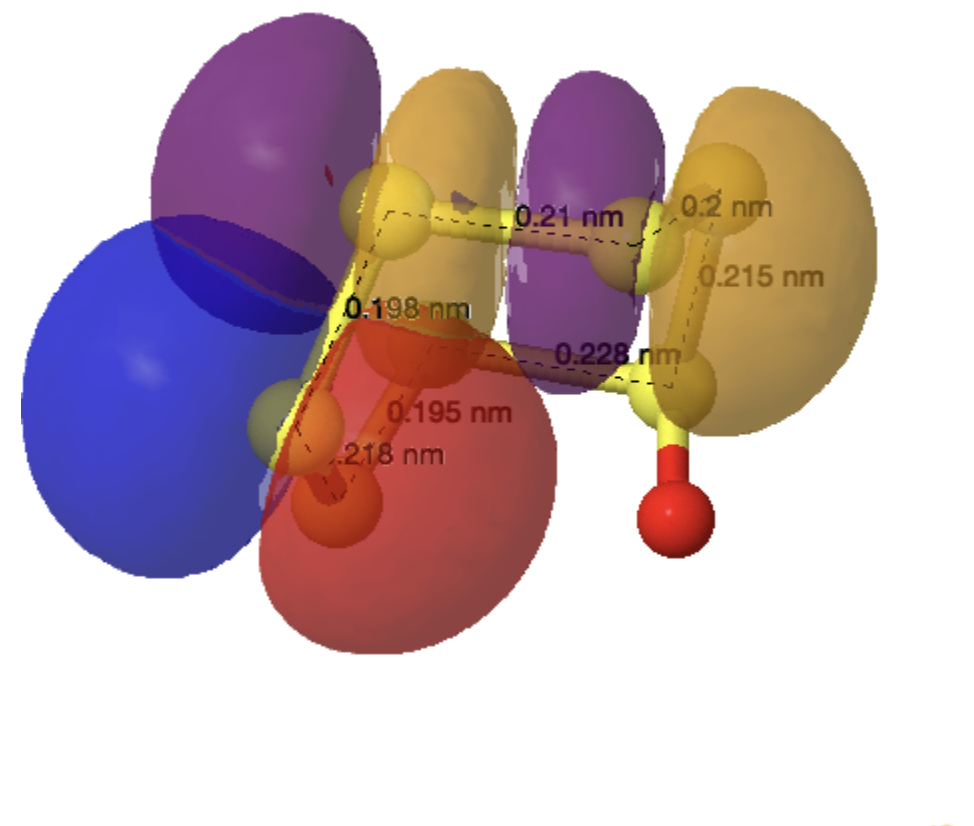
The monosulfoxide of cyclo-heptasulfur was reported along with cycloheptasulfur itself in 1977,[1] along with the remarks that “The δ modification of S7 contains bonds of widely differing length: this has never been observed before in an unsubstituted molecule. and “the same effect having also been observed in other sulfur rings (S8O, S7I1+ and S7O).” Here I take a look at the last of these other molecules, the monosulfoxide of S7, as a follow up to the commentary on S7 itself.[2]
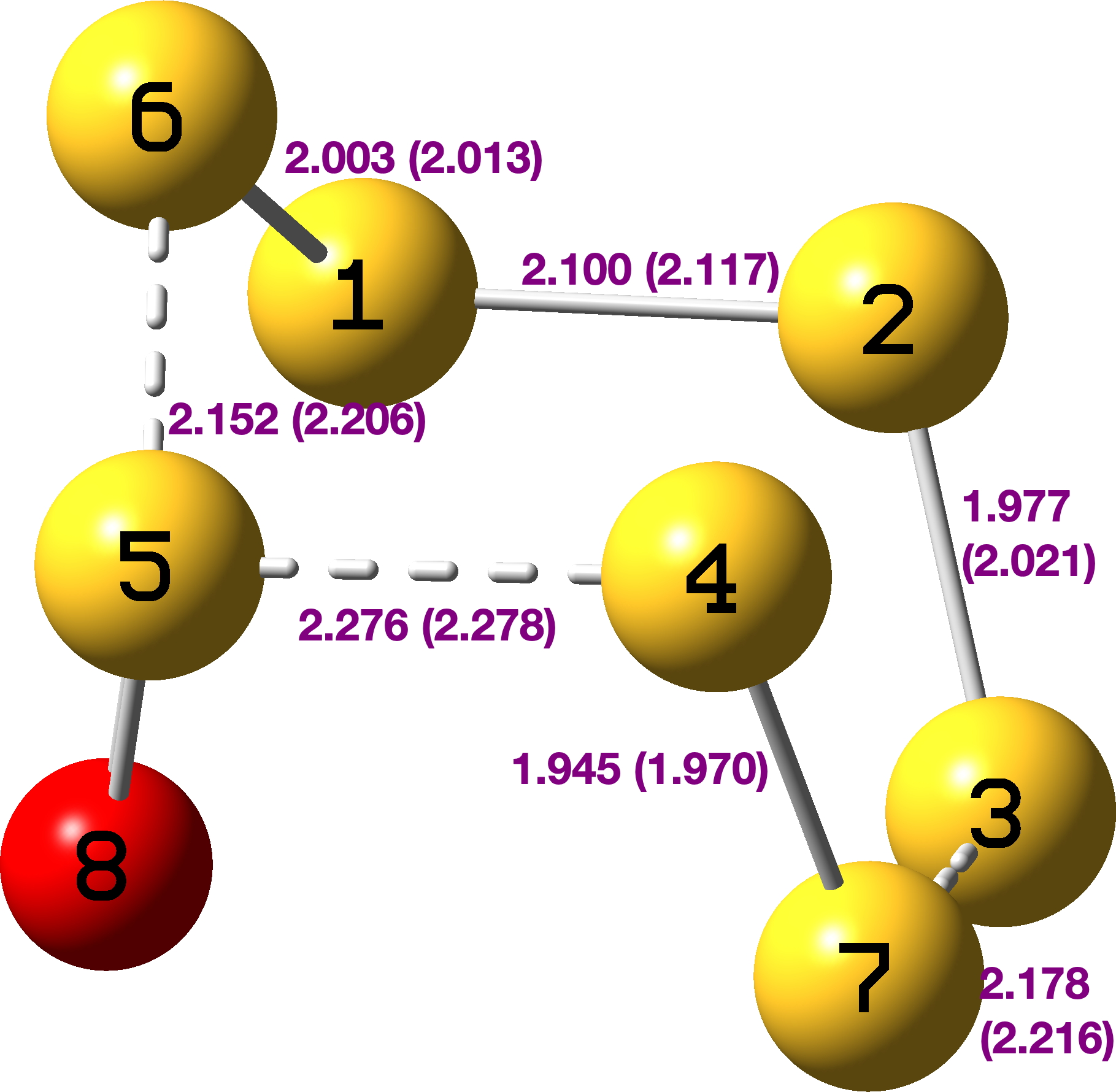
The axial oxygen isomer is calculated as being 3.68 kcal/mol more stable than the equatorial form[3] and a comparison of its calculated (MN15L/Def2-TZVPP) and observed structure is shown below. The S-S lengths do indeed vary widely.
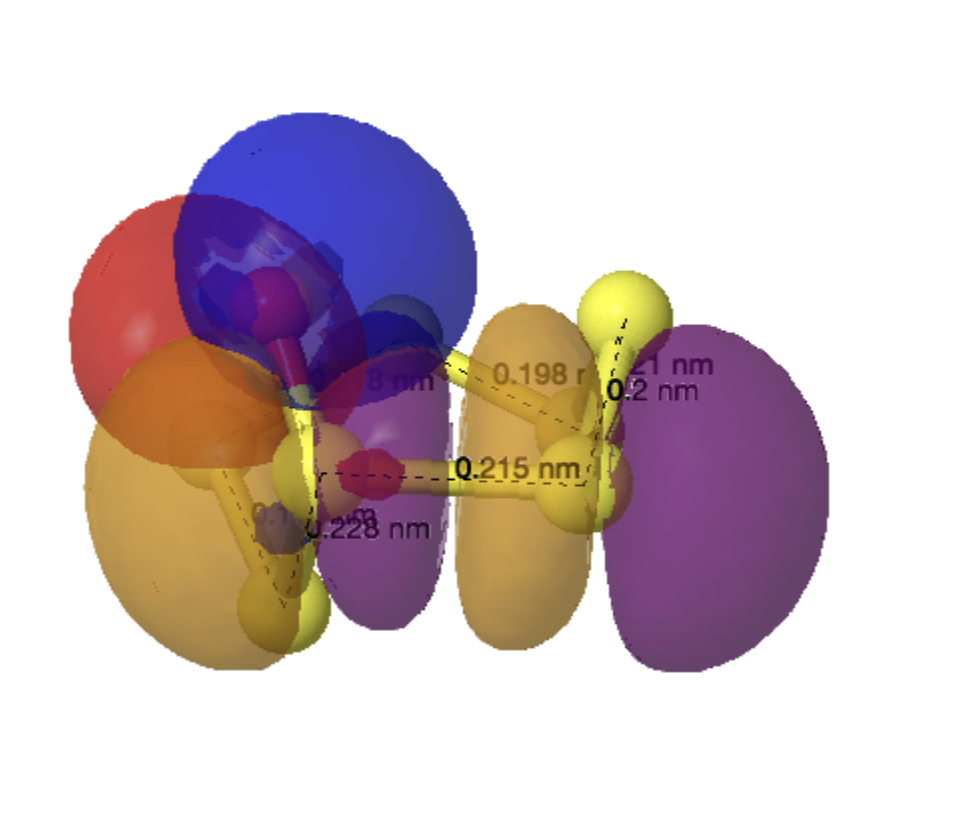
As before, an explanation is provided by analysing the orbitals of the molecule using NBO7. The interactions tabled below are ordered by the largest first. That from the oxygen into the S4-S5 antibonding NBO (28.2 kcal/mol) is the biggest I have observed for an anomeric effect involving an S-S bond. The greatest all-sulfur effect (16.8 kcal/mol) is increased compared to that previously found for S7 itself (12.35 kcal/mol).
| Donor lone Pair | Acceptor antibonding NBO | E(2), kcal/mol | Acceptor bond distance, Å |
| O8 | S4-S5 | 28.2 | 2.28 |
| O8 | S5-S6 | 20.2 | 2.15 |
| S7 | S4-S5 | 16.8 | 2.28 |
| S4 | S3-S7 | 14.8 | 2.18 |
| S2 | S3-S7 | 12.5 | 2.18 |
| S3 | S5-S6 | 10.3 | 2.15 |
| O8 | S5-S6 | 9.6 | 2.15 |
| S6 | S1-S2 | 9.1 | 2.10 |
| E(2) | NBO overlaps‡ Click on image to load 3D rotatable model |
| 28.2 |
|
| 20.0 |
|
| 16.8 |
|
| 14.8 |
|
| 12.5 |
|
| 10.3 |
|
| 9.6 |
|
| 9.1 |
|
The S-S stretching modes also vary by more than a factor of two; ν4-7 619 cm-1, ν2-3 528 cm-1, ν1-6 548 cm-1, ν3-7 368 cm-1, ν5-6 331 cm-1, ν4-5 287 cm-1.

It is indeed remarkable that this small molecule can exhibit as many as eight different anomeric interactions, including two unusually large ones and three regular ones. The result is the profusion of different S-S bond lengths originally commented[1] on accompanied by the wide variety of S-S stretching modes. Can this record be beaten, either in the number or the magnitude of the effects. The answer is YES, but not for a known molecule. See next post!
References
- R. Steudel, R. Reinhardt, and T. Sandow, "Bond Interaction in Sulfur Rings: Crystal and Molecular Structure of <i>cyclo</i>‐Heptasulfur Oxide, S<sub>7</sub>O", Angewandte Chemie International Edition in English, vol. 16, pp. 716-716, 1977. https://doi.org/10.1002/anie.197707161
- H. Rzepa, "Cyclo-Heptasulfur, S<sub>7</sub> – a classic anomeric effect discovered during a pub lunch!", 2025. https://doi.org/10.59350/rzepa.28407
- H. Rzepa, "Cyclo-Heptasulfur, S7 – a classic anomeric effect discovered during a pub lunch!", 2025. https://doi.org/10.14469/hpc/15228