I am at the ACS meeting, attending a session on chemistry and the Internet. This post was inspired by Chemicalize, a service offered by ChemAxon, which scans a post like this one, and identifies molecules named. I had previously used generic post taggers, which frankly did not work well in identifying chemical content. So this is by way of an experiment. I list below some of the substances about which I have blogged, to see how the chemicalizer works. (more…)
Archive for March, 2011
Chemicalizing a blog.
Wednesday, March 30th, 2011From the colour blue to molecular wires
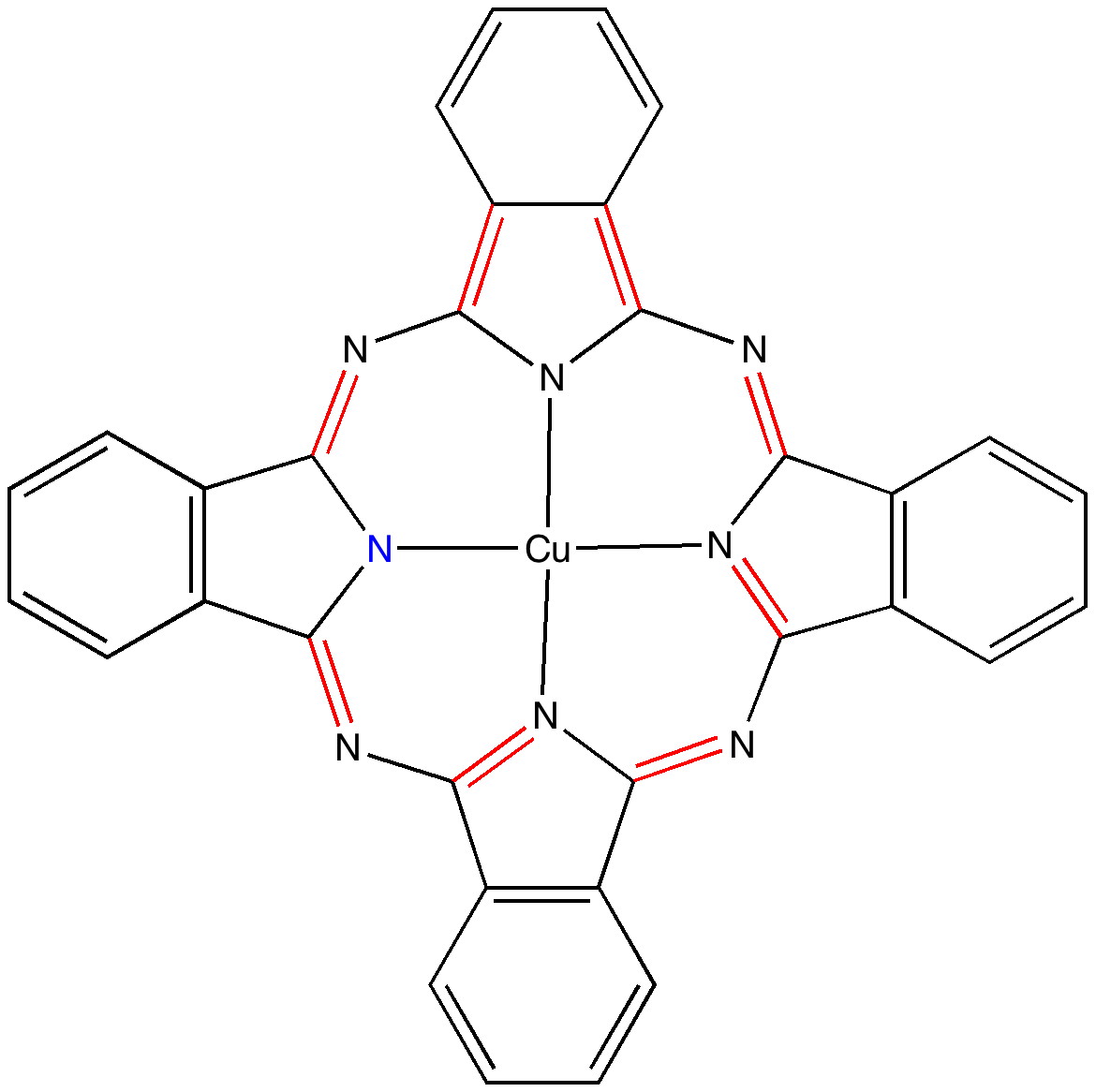
Wednesday, March 9th, 2011In the previous post I pondered the colour of Monastral blue (copper phthalocyanine). Something did not quite fit, and so I speculated that perhaps some oxidation of the pigment might give a new species. This species (Cambridge code FEGJOQ) comprises two parts of copper phthalocyanine, 1 part of the corresponding cation, and 1 part of triodide anion. Looking at the packing of this system, I spotted something I had seen some time ago in NaI2.Acetone, namely an infinitely long and absolutely straight chain of iodine atoms, a molecular wire if you like.
Monastral: the colour of blue
Tuesday, March 8th, 2011The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else. Fortunately, much of that story is actually recorded on film (itself a unique archive dating from 1933 and being one of the very first colour films in existence!). Patrick Linstead, a young chemist then (he eventually rose to become rector of Imperial College) tells the story himself here. It is well worth watching, if only for its innocent social commentary on the English class system (and an attitude to laboratory safety that should not be copied nowadays). Here I will comment only on its colour and its aromaticity.
The thermodynamic energies of left and right handed DNA.
Saturday, March 5th, 2011In this earlier post, I noted some aspects of the calculated structures of both Z- and B-DNA duplexes. These calculations involved optimising the positions of around 250-254 atoms, for d(CGCG)2 and d(ATAT)2, an undertaking which has taken about two months of computer time! The geometries are finally optimised to the point where 2nd derivatives can be calculated, and which reveal up to 756 all-positive force constants and 6 translations and rotations which are close to zero! This now lets one compute the thermodynamic relative energies using ωB97XD/6-31G(d) (for 2nd derivatives) and 6-31G(d,p) (for dispersion terms). All geometries are optimized using a continuum solvent field (water), and are calculated, without a counterion, as hexa-anions. (more…)
Lapis lazuli: the colour of ultramarine.
Saturday, March 5th, 2011The formation of cyanohydrins: re-writing the text books. ! or ?
Friday, March 4th, 2011Nucleophilic addition of cyanide to a ketone or aldehyde is a standard reaction for introductory organic chemistry. But is all as it seems? The reaction is often represented as below, and this seems simple enough.