The molecule below was characterised in 1996 (DOI: 10.1246/cl.1996.489) and given the name tris(dithiolene)vanadium (IV). No attempt was made in the original article to give this molecule a Lewis structure using Lewis electron pair bonds. This blog will explore some of the issues that arise when this is attempted.1
Archive for September, 2010
Secrets of a university admissions interviewer
Sunday, September 19th, 2010Many university chemistry departments, and mine is no exception, like to invite applicants to our courses to show them around. Part of the activities on the day is an “interview” in which the candidate is given a chance to shine. Over the years, I have evolved questions about chemistry which can form the basis of discussion, and I thought I would share one such question here. It starts by my drawing on the blackboard (yes, I really still use one!) the following molecular structure.
Solid carbon dioxide: hexacoordinate carbon?
Friday, September 17th, 2010Carbon dioxide is much in the news, not least because its atmospheric concentration is on the increase. How to sequester it and save the planet is a hot topic. Here I ponder its solid state structure, as a hint to its possible reactivity, and hence perhaps for clues as to how it might be captured. The structure was determined (DOI 10.1103/PhysRevB.65.104103) as shown below.
The oldest reaction mechanism: updated!
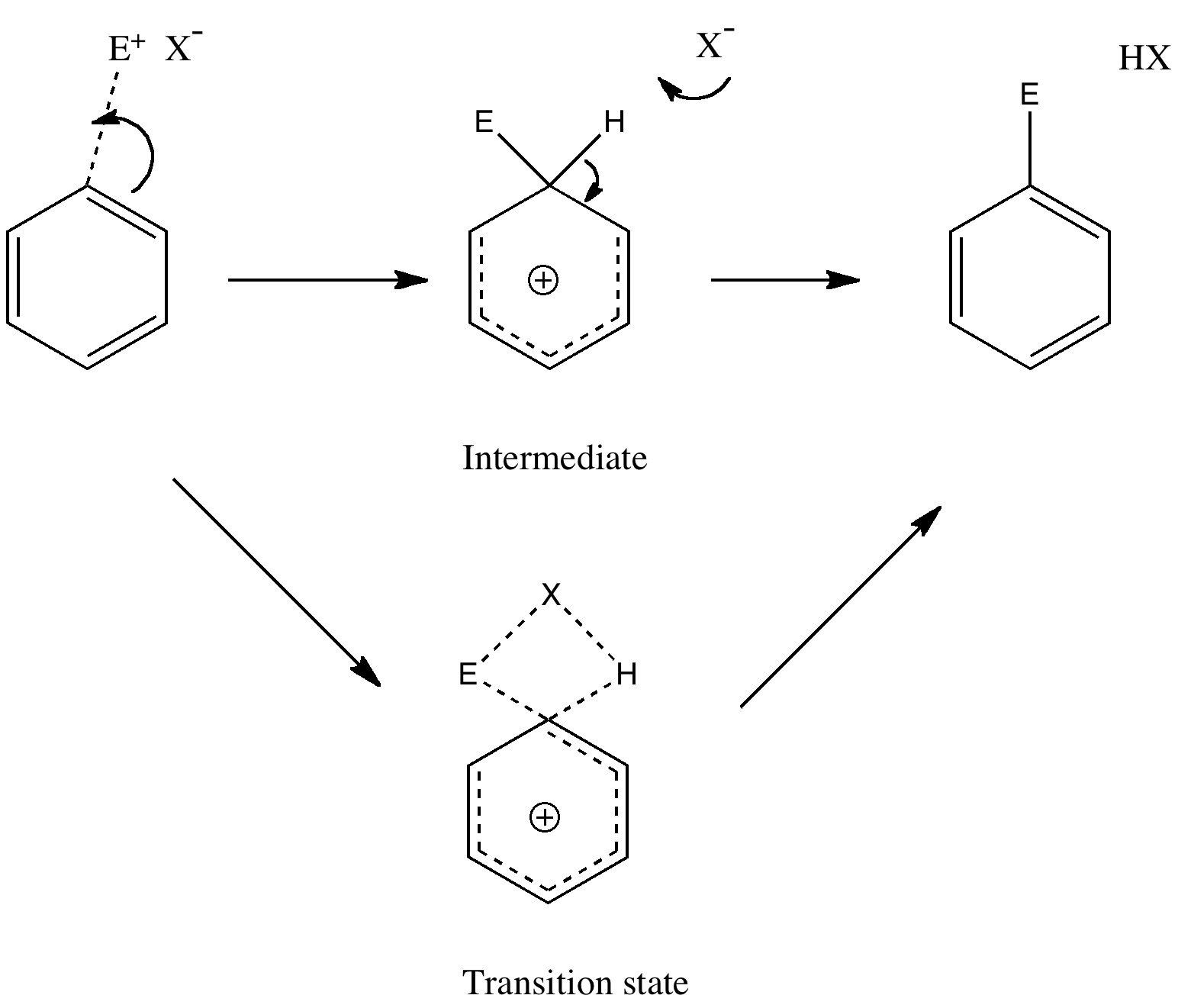
Tuesday, September 14th, 2010Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century. In 1890, Henry Armstrong proposed what amounts to close to the modern mechanism for the process we now know as aromatic electrophilic substitution [1]. Beyond doubt, he invented what is now known as the Wheland Intermediate (about 50 years before Wheland wrote about it, and hence I argue here it should really be called the Armstrong/Wheland intermediate). This is illustrated (in modern style) along the top row of the diagram.
References
- "Proceedings of the Chemical Society, Vol. 6, No. 85", Proceedings of the Chemical Society (London), vol. 6, pp. 95, 1890. https://doi.org/10.1039/pl8900600095