Archive for the ‘Interesting chemistry’ Category
Saturday, February 14th, 2015
According to Guggemos, Slavicek and Kresin, about 5-6![1]. This is one of those simple ideas, which is probably quite tough to do experimentally. It involved blasting water vapour through a pinhole, adding HCl and measuring the dipole-moment induced deflection by an electric field. They found “evidence for a noticeable rise in the dipole moment occurring at n≈5–6“.
(more…)
References
- N. Guggemos, P. Slavíček, and V.V. Kresin, "Electric Dipole Moments of Nanosolvated Acid Molecules in Water Clusters", Physical Review Letters, vol. 114, 2015. https://doi.org/10.1103/physrevlett.114.043401
Tags:energy, gas phase models, Java, pence, similar energy
Posted in Interesting chemistry, reaction mechanism | 1 Comment »
Tuesday, February 10th, 2015
Steganone is an unusual natural product, known for about 40 years now. The assignment of its absolute configurations makes for an interesting, on occasion rather confusing, and perhaps not entirely atypical story. I will start with the modern accepted stereochemical structure of this molecule, which comes in the form of two separately isolable atropisomers.

The first reported synthesis of this system in 1977 was racemic, and no stereochemistry is shown in the article (structure 2).[1] Three years later an “Asymmetric total synthesis of (-)steganone and revision of its absolute configuration” shows how the then accepted configuration (structure 1 in this article) needs to be revised to the enantiomer shown as structure 12 in the article[2] and matching the above representation. The system has continued to attract interest ever since[3],[4],[5],[6], not least because of the presence of axial chirality in the form of atropisomerism. Thus early on it was shown that the alternative atropisomer, the (aS,R,R) configuration initially emerges out of several syntheses, and has to be converted to the (aR,R,R) configuration by heating[3]. One could easily be fooled by such isomerism!
(more…)
References
- D. Becker, L.R. Hughes, and R.A. Raphael, "Total synthesis of the antileukaemic lignan (±)-steganacin", J. Chem. Soc., Perkin Trans. 1, pp. 1674-1681, 1977. https://doi.org/10.1039/p19770001674
- J. Robin, O. Gringore, and E. Brown, "Asymmetric total synthesis of the antileukaemic lignan precursor (-)steganone and revision of its absolute configuration", Tetrahedron Letters, vol. 21, pp. 2709-2712, 1980. https://doi.org/10.1016/s0040-4039(00)78586-8
- E.R. Larson, and R.A. Raphael, "Synthesis of (–)-steganone", J. Chem. Soc., Perkin Trans. 1, pp. 521-525, 1982. https://doi.org/10.1039/p19820000521
- A. Bradley, W.B. Motherwell, and F. Ujjainwalla, "A concise approach towards the synthesis of steganone analogues", Chemical Communications, pp. 917-918, 1999. https://doi.org/10.1039/a900743a
- M. Uemura, A. Daimon, and Y. Hayashi, "An asymmetric synthesis of an axially chiral biaryl via an (arene)chromium complex: formal synthesis of (–)-steganone", J. Chem. Soc., Chem. Commun., vol. 0, pp. 1943-1944, 1995. https://doi.org/10.1039/c39950001943
- B. Yalcouye, S. Choppin, A. Panossian, F.R. Leroux, and F. Colobert, "A Concise Atroposelective Formal Synthesis of (–)‐Steganone", European Journal of Organic Chemistry, vol. 2014, pp. 6285-6294, 2014. https://doi.org/10.1002/ejoc.201402761
Tags:free energy, lowest energy conformation, natural product, simulation, spectroscopy, unusual natural product, X-ray
Posted in Interesting chemistry | No Comments »
Friday, January 23rd, 2015
Sometimes you come across a bond in chemistry that just shouts at you. This happened to me in 1989[1] with the molecule shown below. Here is its story and, 26 years later, how I responded.
(more…)
References
- P. Camilleri, C.A. Marby, B. Odell, H.S. Rzepa, R.N. Sheppard, J.J.P. Stewart, and D.J. Williams, "X-Ray crystallographic and NMR evidence for a uniquely strong OH ? N hydrogen bond in the solid state and solution", Journal of the Chemical Society, Chemical Communications, pp. 1722, 1989. https://doi.org/10.1039/c39890001722
Tags:energy, high chemical shifts, New Hampshire, unusual chemical shift
Posted in Historical, Interesting chemistry | No Comments »
Sunday, December 7th, 2014
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[1]. 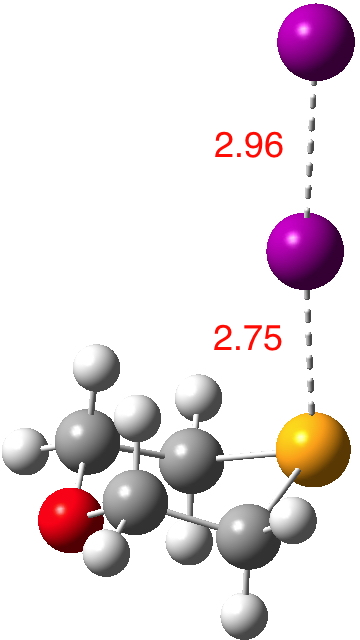 The features of interest include:
The features of interest include:
(more…)
References
- H. Maddox, and J.D. McCullough, "The Crystal and Molecular Structure of the Iodine Complex of 1-Oxa-4-selenacyclohexane, C<sub>4</sub>H<sub>8</sub>OSe.I<sub>2</sub>", Inorganic Chemistry, vol. 5, pp. 522-526, 1966. https://doi.org/10.1021/ic50038a006
Tags:chair, crystal structure search
Posted in crystal_structure_mining, Interesting chemistry | 7 Comments »
Sunday, November 30th, 2014
Pursuing the topic of halogen bonds, the system DABCO (a tertiary dibase) and iodine form an intriguing complex. Here I explore some unusual features of the structure HEKZOO[1] as published in 2012[2] and ask whether the bonding between the donor (N) and the acceptor (I-I) really is best described as a “non-covalent-interaction” (NCI) or not.
(more…)
References
- Peuronen, A.., Valkonen, A.., Kortelainen, M.., Rissanen, K.., and Lahtinen, M.., "CCDC 879935: Experimental Crystal Structure Determination", 2013. https://doi.org/10.5517/ccyjn03
- A. Peuronen, A. Valkonen, M. Kortelainen, K. Rissanen, and M. Lahtinen, "Halogen Bonding-Based “Catch and Release”: Reversible Solid-State Entrapment of Elemental Iodine with Monoalkylated DABCO Salts", Crystal Growth & Design, vol. 12, pp. 4157-4169, 2012. https://doi.org/10.1021/cg300669t
Tags:bond energy, co-operative, donor-acceptor interaction energy
Posted in Interesting chemistry | 5 Comments »
Saturday, November 29th, 2014
Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
(more…)
Tags:crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »
Thursday, October 9th, 2014
This second report highlights two “themes”, or common ideas that seem to emerge spontaneously from diversely different talks. Most conferences do have them.
(more…)
Tags:Complex Biological Systems, condensation, gas-phase molecular species, metal surface catalysis, molecular systems, non-crystalline systems, organic chemist, organometallic systems, potential energy surface, representative, Stefan Grimme, Thus Emily Carter
Posted in Interesting chemistry, WATOC reports | 1 Comment »
Monday, October 6th, 2014
I am attending a conference. Plenaries at such events can sometimes provide interesting pointers on things to come (and sometimes they simply point to things past). At WATOC2014 in Santiago Chile, the first plenary was by Paul Ayers with the impressive title “Concepts for organising chemical knowledge” which certainly sounds as if it is pointing forward!
(more…)
Tags:chemical knowledge, chemical philosophy, chemical rules, Chile, Paul Ayers, Paul Geerlings, Santiago, World Association of Theoretical and cOmputational Chemists
Posted in Interesting chemistry | No Comments »
Monday, August 4th, 2014
The outcome of pericyclic reactions con depend most simply on three conditions, any two of which determine the third. Whether the catalyst is Δ or hν (heat or light), the topology determining any stereochemistry and the participating electron count (4n+2/4n). It is always neat to conjure up a simple switch to toggle these; heat or light is simple, but what are the options for toggling the electron count? Here is one I have contrived by playing a game with the periodic table.  The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2]
The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2] 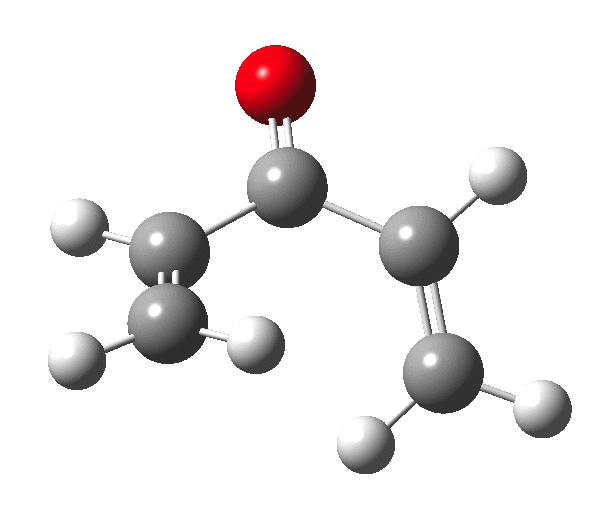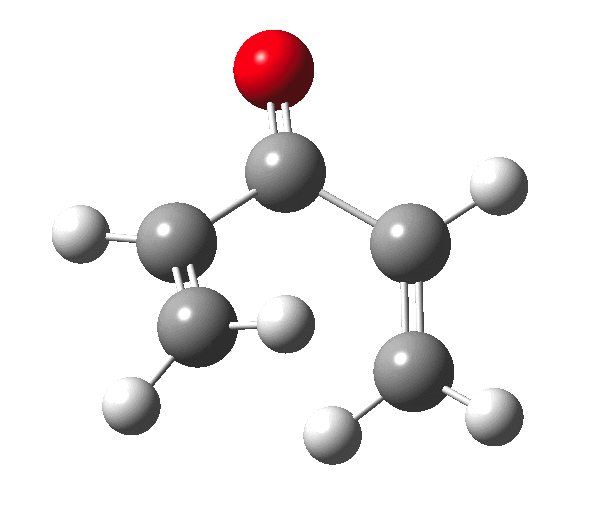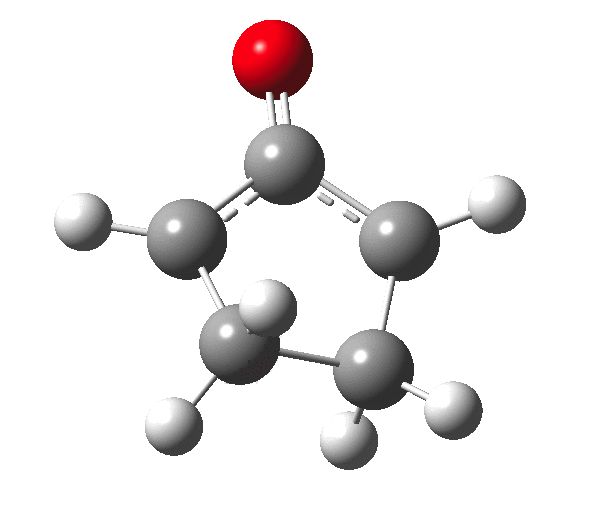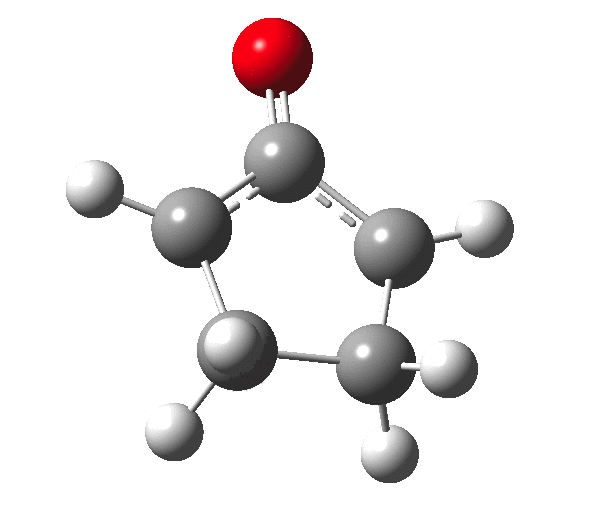

 So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡
So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡ 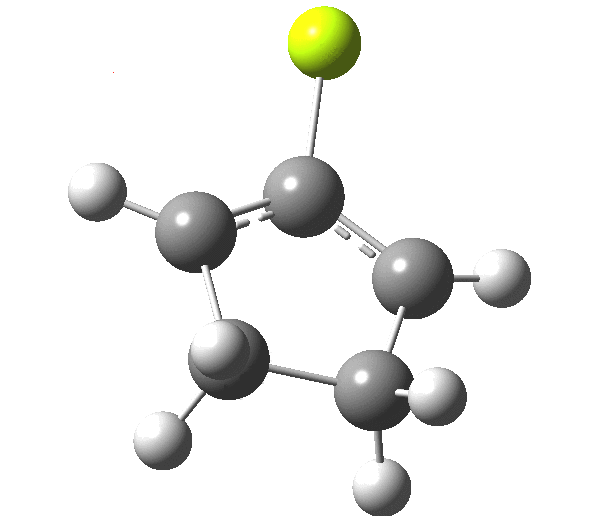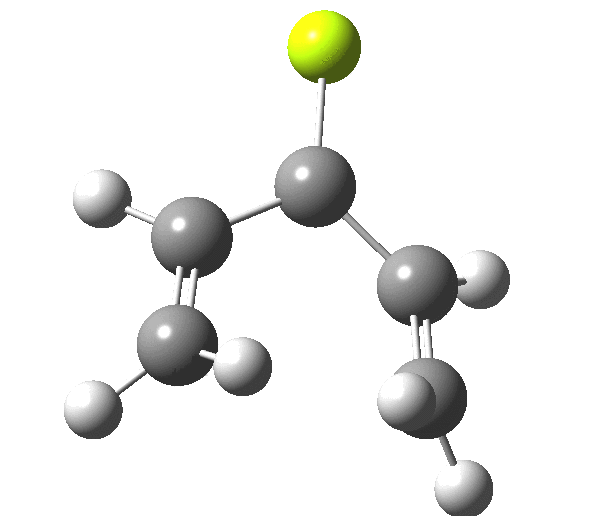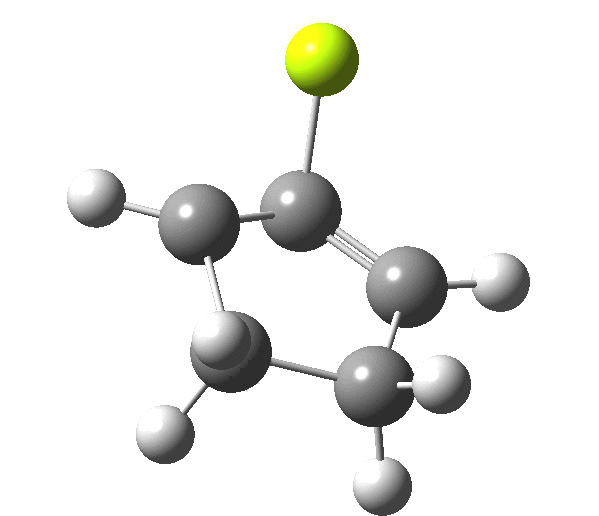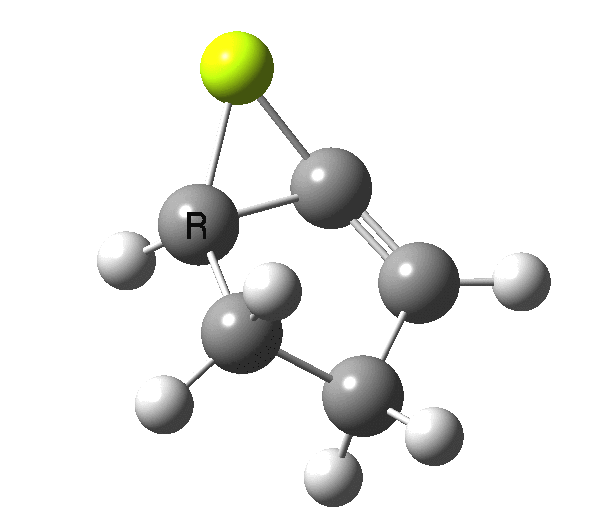
 This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.
This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.
(more…)
References
- H.S. Rzepa, "Gaussian Job Archive for C5H6O", 2014. https://doi.org/10.6084/m9.figshare.1125721
- H.S. Rzepa, "Gaussian Job Archive for C5H6BF3O", 2014. https://doi.org/10.6084/m9.figshare.1125724
- H.S. Rzepa, "Gaussian Job Archive for C5H6Be", 2014. https://doi.org/10.6084/m9.figshare.1125792
Tags:animation
Posted in Interesting chemistry, pericyclic | No Comments »



Halogen bonds: Part 1.
Saturday, November 29th, 2014Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
(more…)
Tags:crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »