Archive for the ‘Interesting chemistry’ Category
Saturday, July 19th, 2014
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B40 to be specific.[1] My interest was in how the σ and π-electrons were partitioned. In a C40, one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that. Having one electron less per atom, one might imagine that a fullerene-like boron cluster would have no π-electrons. But the element has a propensity[2] to promote its σ-electrons into the π-manifold, leaving a σ-hole. So how many π-electrons does B40 have? These sorts of clusters are difficult to build using regular structure editors, and so coordinates are essential. The starting point for a set of coordinates with which to compute a wavefunction was the supporting information. Here is the relevant page: 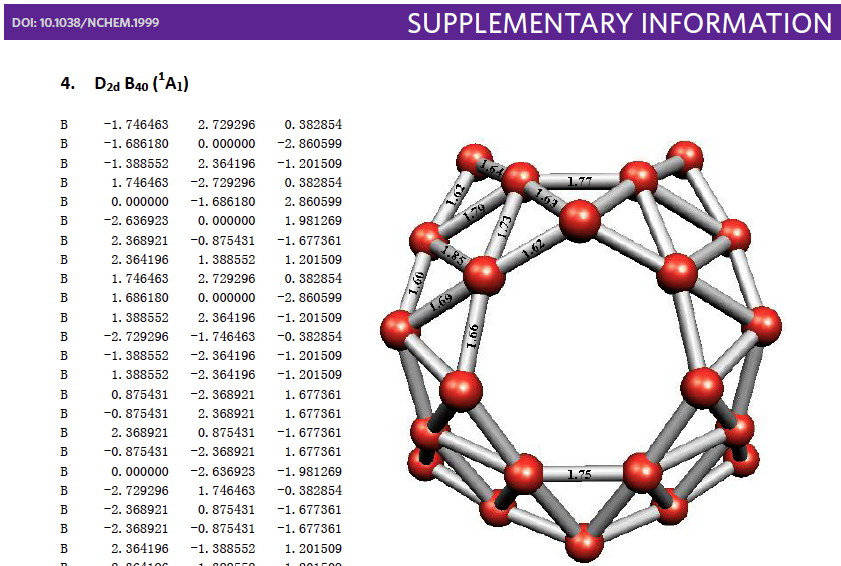 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
(more…)
References
- H. Zhai, Y. Zhao, W. Li, Q. Chen, H. Bai, H. Hu, Z.A. Piazza, W. Tian, H. Lu, Y. Wu, Y. Mu, G. Wei, Z. Liu, J. Li, S. Li, and L. Wang, "Observation of an all-boron fullerene", Nature Chemistry, vol. 6, pp. 727-731, 2014. https://doi.org/10.1038/nchem.1999
- H.S. Rzepa, "The distortivity of π-electrons in conjugated boron rings", Physical Chemistry Chemical Physics, vol. 11, pp. 10042, 2009. https://doi.org/10.1039/b911817a
Tags:Acrobat, Adobe, chemical markup, DOS, operating systems, PDF, pence, Unix
Posted in Chemical IT, Interesting chemistry | 2 Comments »
Thursday, June 26th, 2014
The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack.  My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder). 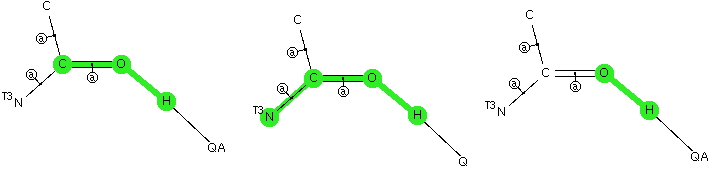 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 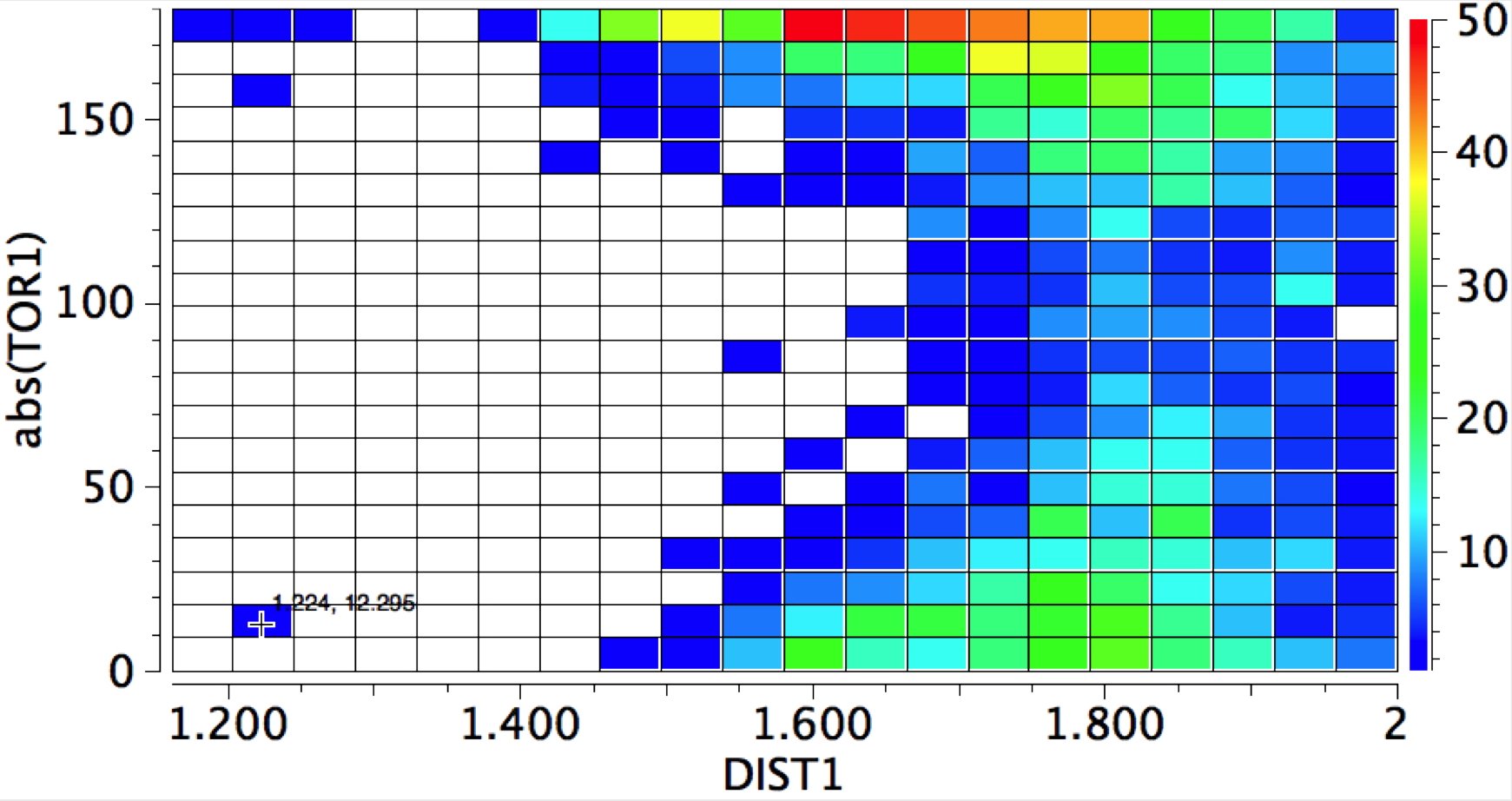
(more…)
Tags:electronics, energy, metal results, metal-π-bond interactions, search definition
Posted in crystal_structure_mining, General, Interesting chemistry | No Comments »
Friday, June 6th, 2014
Following the discussion here of Kekulé’s suggestion of what we now call a vibrational mode (and which in fact now bears his name), I thought I might apply the concept to a recent molecule known as [2.2]paracyclophane. The idea was sparked by Steve Bachrach’s latest post, where the “zero-point” structure of the molecule has recently been clarified as having D2 symmetry.[1]
(more…)
References
- H. Wolf, D. Leusser, M. Jørgensen, R. Herbst‐Irmer, Y. Chen, E. Scheidt, W. Scherer, B.B. Iversen, and D. Stalke, "Phase Transition of [2,2]‐Paracyclophane – An End to an Apparently Endless Story", Chemistry – A European Journal, vol. 20, pp. 7048-7053, 2014. https://doi.org/10.1002/chem.201304972
Tags:10.1002, 201304972, Hiberty and co, Steve Bachrach
Posted in Historical, Interesting chemistry | No Comments »
Sunday, April 20th, 2014
Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.
(more…)
Tags:1M solutions, carbon fixation, chair, chemist, energy, free energy, low energy, low energy sink, lower energy conformation, lower energy isomer, Peter Medawar, phosphate
Posted in Interesting chemistry, reaction mechanism | 1 Comment »
Tuesday, April 15th, 2014
The journal of chemical education can be a fertile source of ideas for undergraduate student experiments. Take this procedure for asymmetric epoxidation of an alkene.[1] When I first spotted it, I thought not only would it be interesting to do in the lab, but could be extended by incorporating some modern computational aspects as well.
(more…)
References
- A. Burke, P. Dillon, K. Martin, and T.W. Hanks, "Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", Journal of Chemical Education, vol. 77, pp. 271, 2000. https://doi.org/10.1021/ed077p271
Tags:chemical education, energy, free energy, Shi Fructose
Posted in Interesting chemistry, reaction mechanism | 2 Comments »
Sunday, April 13th, 2014
Around 100 tons of the potent antimalarial artemisinin is produced annually; a remarkable quantity given its very unusual and fragile looking molecular structure (below). When I looked at this, I was immediately struck by a thought: surely this is a classic molecule for analyzing stereoelectronic effects (anomeric and gauche). Here this aspect is explored.
(more…)
Tags:C7 Lp, Cambridge, stereo-electronics
Posted in crystal_structure_mining, Interesting chemistry | No Comments »
Wednesday, March 12th, 2014
My previous post related to the aromatic electrophilic substitution of benzene using as electrophile phenyl diazonium chloride. Another prototypical reaction, and again one where benzene is too inactive for the reaction to occur easily, is the catalyst-free bromination of benzene to give bromobenzene and HBr.
(more…)
Tags:activation energy, animation, aromatic, Boris Galabov, co-author, electrophilic, lowest energy solution, o/p director of aromatic electrophilic substitution, Paul Schleyer, pence, substitution
Posted in Interesting chemistry, reaction mechanism | 8 Comments »
Sunday, February 9th, 2014
This is the time of year when I deliver two back-2-back lecture courses, and yes I do update and revise the content! I am always on the look-out for nice new examples that illustrate how concepts and patterns in chemistry can be joined up to tell a good story. My attention is currently on conformational analysis; and here is an interesting new story to tell about it.
(more…)
Posted in General, Interesting chemistry | 2 Comments »
 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
