To quote from Wikipedia: in chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The most ubiquitous type of carbene of recent times is the one shown below as 1, often referred to as a resonance stabilised or persistent carbene. This type is of interest because of its ability to act as a ligand to an astonishingly wide variety of metals, with many of the resulting complexes being important catalysts. The Wiki page on persistent carbenes shows them throughout in form 1 below, thus reinforcing the belief that they have a valence of two and by implication six (2×2 shared + 2 unshared) electrons in the valence shell of carbon. Here I consider whether this name is really appropriate.
Archive for the ‘crystal_structure_mining’ Category
A periodic table for anomeric centres.
Saturday, August 6th, 2016In the last few posts, I have explored the anomeric effect as it occurs at an atom centre X. Here I try to summarise the atoms for which the effect is manifest in crystal structures.
Stereoelectronic effects galore: bis(trifluoromethyl)trioxide.
Thursday, August 4th, 2016Here is a little molecule that can be said to be pretty electron rich. There are lots of lone pairs present, and not a few electron-deficient σ-bonds. I thought it might be fun to look at the stereoelectronic interactions set up in this little system.
Anomeric effects at carbon involving lone pairs originating from one or two nitrogens.
Friday, July 8th, 2016The previous post looked at anomeric effects set up on centres such as B, Si or P, and involving two oxygen groups attached to these atoms. Here I vary the attached groups to include either one or two nitrogen atoms.[1]
References
- H. Rzepa, "Anomeric effects at carbon, involving lone pairs originating from one or two nitrogens", 2016. https://doi.org/10.14469/hpc/936
Anomeric effects at boron, silicon and phosphorus.
Friday, July 1st, 2016
The anomeric effect occurs at 4-coordinate (sp3) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon. I start with 4-coordinate boron, using the crystal structure search definition below (along with R < 0.1, no disorder, no errors).[1]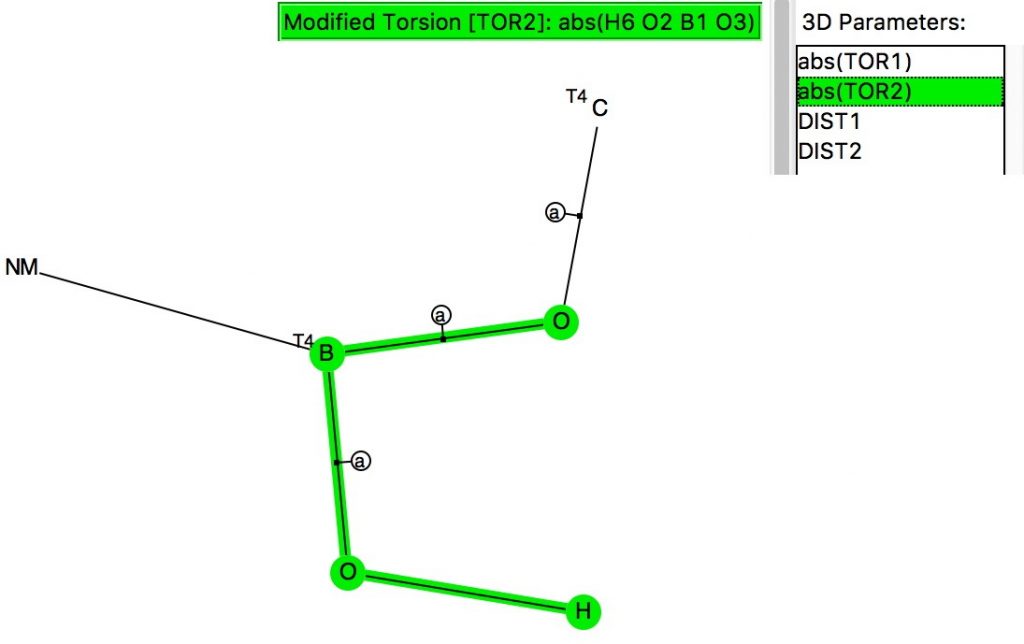
References
- H. Rzepa, "Anomeric effects at boron, silicon and phosphorus.", 2016. https://doi.org/10.14469/hpc/696
How does an OH or NH group approach an aromatic ring to hydrogen bond with its π-face?
Wednesday, June 22nd, 2016I previously used data mining of crystal structures to explore the directing influence of substituents on aromatic and heteroaromatic rings. Here I explore, quite literally, a different angle to the hydrogen bonding interactions between a benzene ring and OH or NH groups.
Exploring the electrophilic directing influence of heteroaromatic rings using crystal structure data mining.
Tuesday, June 21st, 2016This is a follow-up to the post on exploring the directing influence of (electron donating) substituents on benzene[1] with the focus on heteroaromatic rings such indoles, pyrroles and group 16 analogues (furans, thiophenes etc).
References
- H.S. Rzepa, "Discovering More Chemical Concepts from 3D Chemical Information Searches of Crystal Structure Databases", Journal of Chemical Education, vol. 93, pp. 550-554, 2015. https://doi.org/10.1021/acs.jchemed.5b00346
Why is the carbonyl IR stretch in an ester higher than in a ketone: crystal structure data mining.
Saturday, June 18th, 2016In this post, I pondered upon the C=O infra-red spectroscopic properties of esters, and showed three possible electronic influences:
A wider look at chlorine trifluoride: crystal structures and data mining.
Friday, June 10th, 2016A while ago, I explored how the 3-coordinate halogen compound ClF3 is conventionally analyzed using VSEPR (valence shell electron pair repulsion theory). Here I (belatedly) look at other such tri-coordinate halogen compounds using known structures gleaned from the crystal structure database (CSD).