Opportunities
Places available within the Kucernak research group are advertised here. This page is updated as new positions become available. Please apply by sending an email to Anthony.Post Doctoral Research
We currently have no advertised positions for post-doc research within the group, however please feel free to send speculative CV's to Anthony.PhD Studentships
We currently have three studentships available, example projects are provided below.Reducing platinum usage in fuel cells: nanoengineering the morphology and activity of fuel cell electrodes
Start Date: 2014
Is there enough platinum in the world to support fuel cells?
Even if there is, will the cost of fuel cells be too much?
Recent work has shown that the performance of catalysts in fuel cells need to increase by about a factor of 3-5 in order to make the precious usage in a fuel cell vehicle 'neutral', that is, the amount of precious metal in the catalytic convertor matches that in the new fuel cell vehicle, so that no extra precious metal is needed. There are a number of ways we can do this e.g. by increasing the performance of the catalyst, or by optimising where current catalysts are placed.
What would the 'perfect' fuel cell electrode look like? It would be an electrode in which transport of reactants and products, electrons and ions was optimised, Figure 1. By producing such an electrode, it would be possible to measure the intrinsic catalyst activity over the entire operational parameter space that exists within a fuel cell (potential, reactant conditions, temperature etc). Furthermore, such an electrode would give valuable insights into the design of commercially relevant electrode structures.
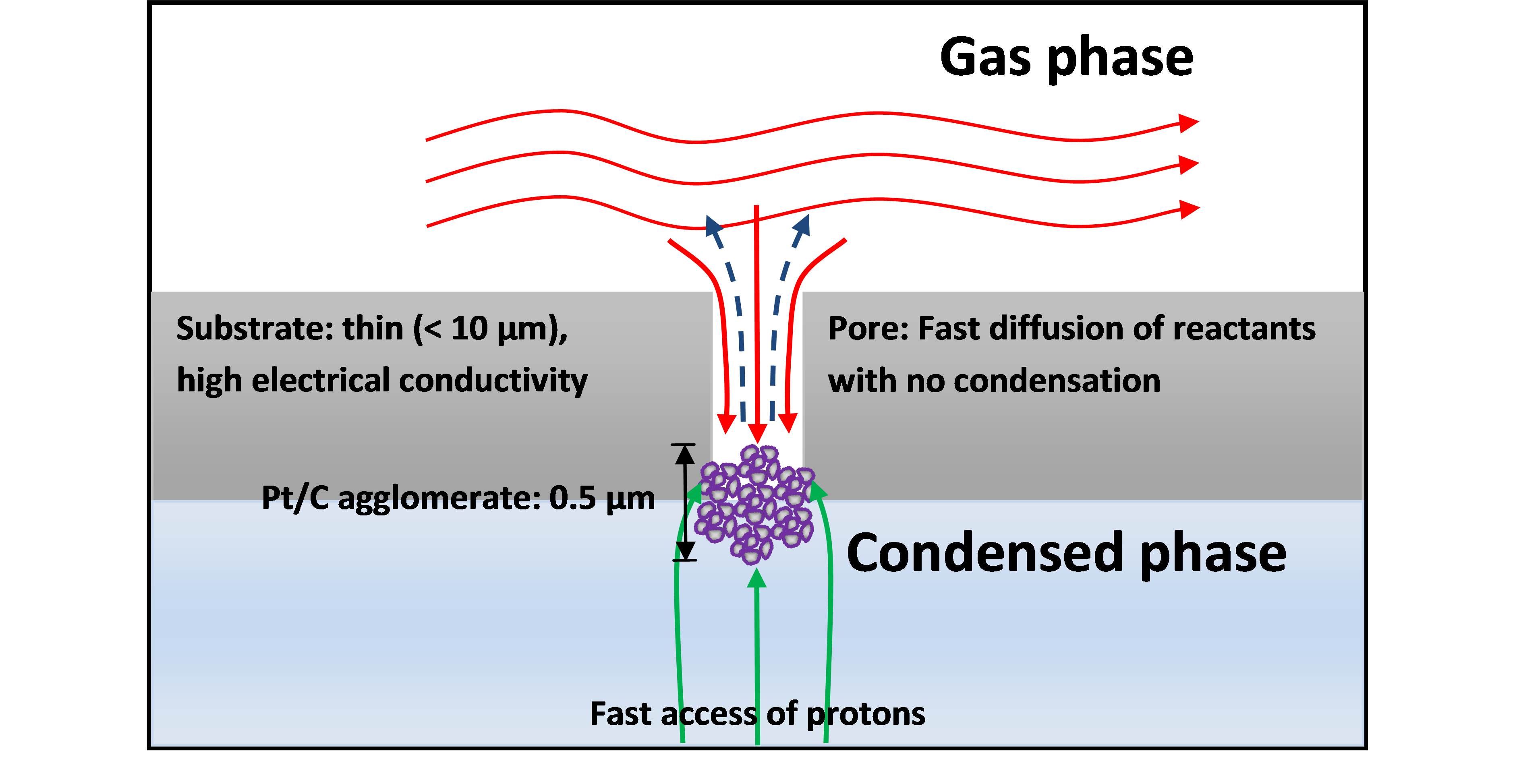
Our successful approach allows us to deposit catalyst particles in the manner suggested in Figure 1. The approach allows uniform catalyst distribution at ultra-low loadings of down to less than 0.2 mgPt cm-2 : more than 1000 lower than used in typical fuel cell electrodes. We estimate that the mass transport rates for these electrodes are in excess of 50 cm s-1. Such high mass transport rates cannot be achieved in current fuel cells.
These electrodes have truly outstanding performance values. For instance, in looking at the hydrogen evolution reaction corresponds we can drive our catalyst to produce 19,000 H2 molecules per platinum surface atom per second.
This PhD project will take this innovative new method and apply it to a number of catalysts to determine fundamental aspects of the oxygen reduction and evolution reactions, and hydrogen oxygen and evolution reactions. These results will then be used in designing better fuel cells and water electrolysers.
The project would suit someone with a good physical sciences or engineering background with some electrochemical knowledge and an interest in 'hands-on' experimentation.
A replacement for platinum: new nitrogen containing carbon compounds which show surprising activity for oxygen reduction
Start Date: 2014
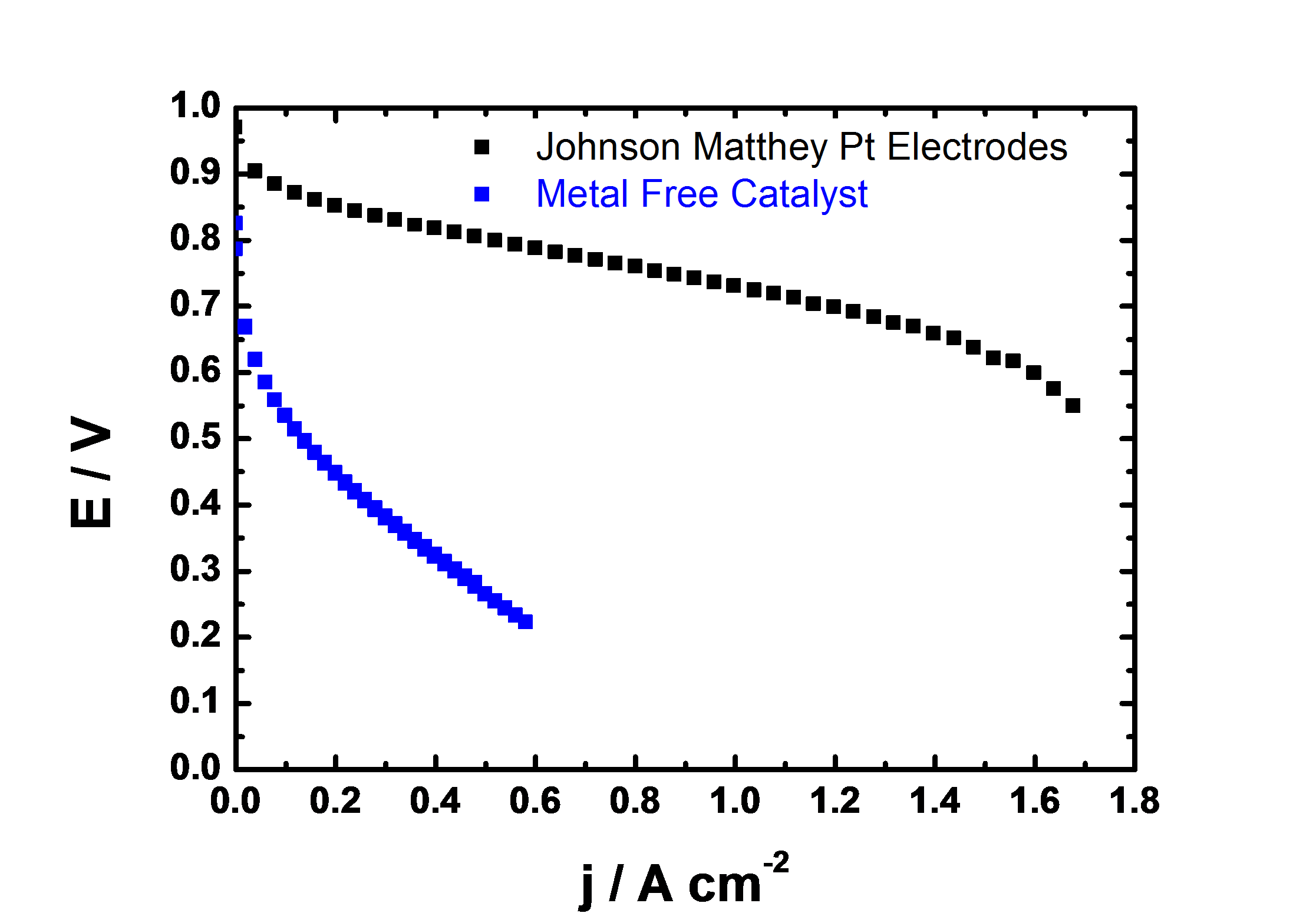
We have recently discovered a new class of nitrogen containing carbon compounds which are surprisingly active towards oxygen reduction, figure 1. One of the remarkable features of this catalyst is that it contains no transition metals, yet is only about 50x less active than platinum on a per-mass basis, Figure 1. Such high activity means that these catalysts may supplant platinum for use in fuel cells.
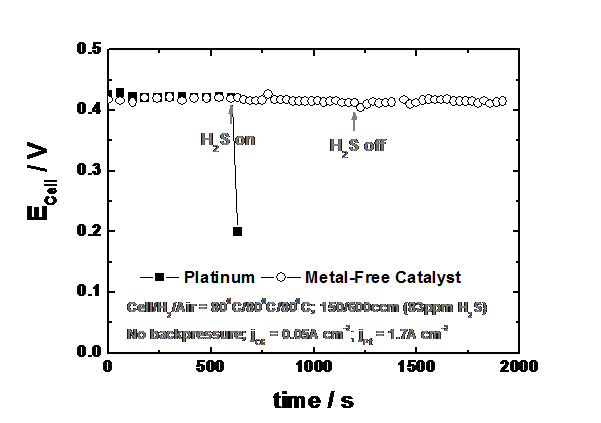
Even more surprising is that the catalyst is totally impervious to the poisons which totally inactivate the platinum catalyst, Figure 2.
Currently, relatively little is known about the active site for the oxygen reduction reaction except that it is correlated with the presence of pyridinic nitrogens embedded in the graphite structure.
The project will involved the following:
- Optimisation of current catalyst materials in order to increase the number of active sites per mass of materials (and thus increase the catalyst performance);
- Experimental work to determine more about the active site including oxygen chemisorption studies and electron microscopy;
- Synthesis of precursors to produce more active catalysts;
- Incorporation of catalysts within fuel cells.
The project would suit someone with a good physical sciences or engineering background with some electrochemical knowledge and an interest in 'hands-on' experimentation.
Low cost fuel cell and redox flow battery (RFB) systems based on a new approach
Start Date: 2014
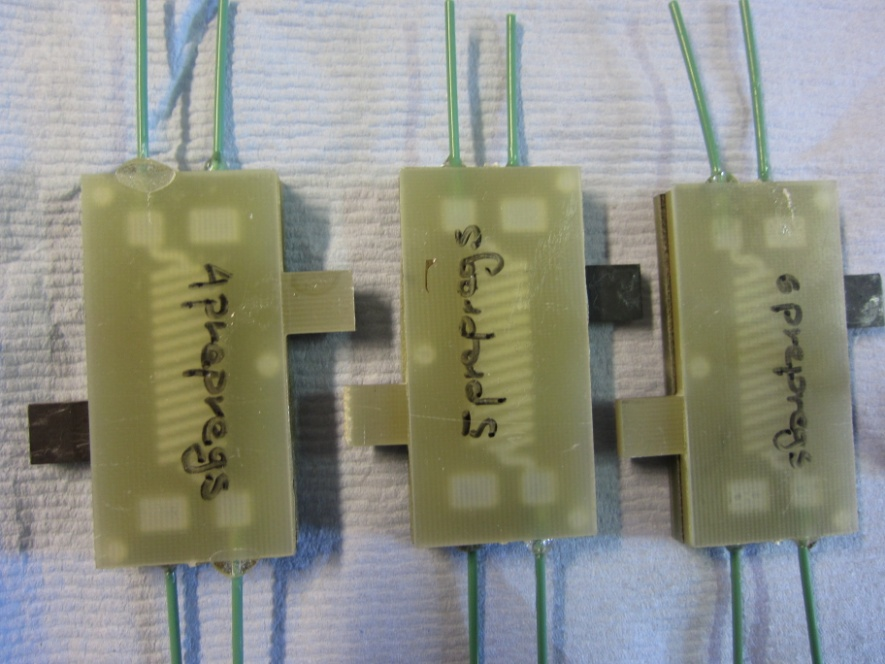
Recent work in our laboratory has shown that it is possible to produce fuel cell systems utilising printed circuitboard technology, an example is shown in figure 1. This has the advantage of significant cost reductions promising mass manufactured systems for less than USD30/kW of power. We have tested these fuel cells and they perform very well, although there are some optimisations which we need to implement in order to improve their performance
 We would like to extend this approach to the area of redox flow batteries (RFBs). These systems may be used to store electrical energy in a liquid energy carrier. As an example a 1kW RFB stack including integrated electronics with electrolyte, pumps etc could provide 8kWh energy storage in a unit the size of a four-drawer filing cabinet. In order to achieve this we will need to do some materials chemistry and produce optimised electrodes based on derivatised carbon aerogels, Figure 2.
We would like to extend this approach to the area of redox flow batteries (RFBs). These systems may be used to store electrical energy in a liquid energy carrier. As an example a 1kW RFB stack including integrated electronics with electrolyte, pumps etc could provide 8kWh energy storage in a unit the size of a four-drawer filing cabinet. In order to achieve this we will need to do some materials chemistry and produce optimised electrodes based on derivatised carbon aerogels, Figure 2.
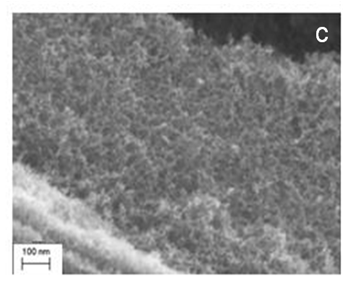
The purpose of this project will be to:
- Produce and test optimised electrodes based on derivatised carbon aerogel structures;
- Look at improved electrolytes for storing energy in RFBs;
- Implement a simple RFB system using similar systems which we have already produced for fuel cells.
The project would suit someone with a good physical sciences or engineering background with some electrochemical knowledge and an interest in 'hands-on' experimentation.