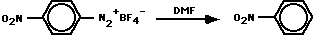
The principal product was nitrobenzene. Decompositions in the prescence of added anisole, nitrobenzene, or iodobenzene gave product mixtures consistent with a p-nitrophenyl radical. The use of DMF, DMF-d1, DMF-d6 and DMF-d7 identified the sites of H-atom abstraction.
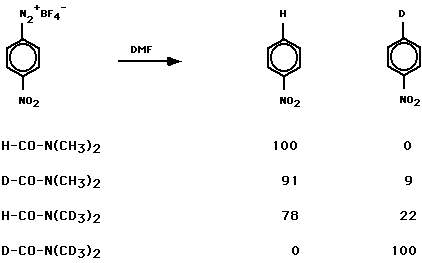 it is consistent with a modest
deuterium kinetic isotope effect in the H-atom abstraction reaction.
it is consistent with a modest
deuterium kinetic isotope effect in the H-atom abstraction reaction.