| [Related articles/posters: 023 005 033 ] |
One of the major aims in supramolecular chemistry consists in mimicking the ability of enzymes to bind to different substrate molecules and to catalyze a stereoselective reaction.
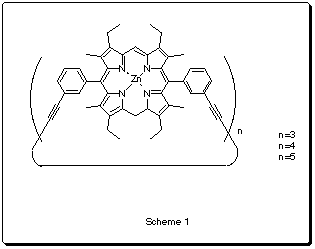
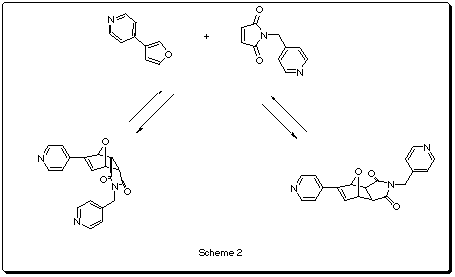
The Sander's group at Cambridge has synthesized some cyclic porphyrine based polymers (Scheme 1) that can act as hosts for different N-donor ligands1. One of them (n=3) has been reported2 to catalyze the exo Diels-Alder reaction between a furan and a maleimide-based dienophile (Scheme2).
The size of these systems makes the modelling by conventional semiempirical methods unfeasible. However, in 1995 Stewart designed a new semiempirical molecular orbital program called MOZYME3 which enables the study of large molecular systems, and despite its name, is not restricted to purely enzymatic systems!
Herein we report our preliminary results in the modelling of the porphyrine trimer catalysts 1 and 2 at the PM3 level and their relative capacities for binding the substrates and products of the Diels-Alder reaction under discussion.
|
|
|
|
Catalyst 1 |
Catalyst 2 |
According to the PM3 results in table 1, in the non-catalytic reaction, the exo-adduct is thermodynamically favored by 3.12 Kcal/mol. The barrier energy for the exo-reaction is lower than the endo by 3.16 Kcal/mol, suggesting that the normal endo specificity for the transition state model is not reproduced in this case. This small difference in the activation energies could explain why at 30oC both products are obtained whereas at 60oC only the exo is isolated (the initially formed endo isomer is converted into the exo2c).
Table 1
|
|
Hf (Kcal/mol) |
|
Hf (Kcal/mol) |
|
Hf (Kcal/mol) |
|
Diene |
26.66 |
endo-adduct |
-4.72 |
TS(endo) |
41.81 |
|
Dienofile |
-18.46 |
exo-adduct |
-7.84 |
TS(exo) |
38.65 |
According to the PM3 calculations (Table 2), the binding energy of the reactants is 19.54 Kcal/mol for the catalyst 1 and 14.4 Kcal/mol for the catalyst 2. These values indicate that both catalysts should be able to complex to the reactants of the reaction, catalyst 1 being probably the better host.
Table 2. PM3 heats of formation in Kcal/mol
|
| ||
|
Catalyst |
849.2 |
695.5 |
|
Complex cat-reactants |
837.9 |
690.8 |
|
Complex cat-exo-adduct |
827.8 |
678.8 |
|
Complex cat-endo-adduct |
832.5 |
684.6 |
The results for catalyst 1 show that the complexed exo-adduct is more stable than the corresponding endo isomer, this stability being substantially enhanced by complexation within the host. In our initial calculations the geometry of the optimized structure showed that the compact endo-adduct was bound to only one of the Zn atoms, resulting in a less stabilizing binding effect (E=838.4 Kcal/mol). Subsequently, we have been able to locate a structure for the bound product which is again less stable than the exo one.
Our initial results in the optimization of the complex catalyst 2-exo- adduct suggested extrusion from the cavity. This resulted to be an artifact of the geometry optimization and we have located a structure in which the adduct fits into the cavity and is lower in energy than the endo. Thus, the complex exo- adduct seems to be more stable than the endo- isomer for both catalyst studied. A further refinement that we have thus far been unable to accomplish, is to fully optimize the transition state structures with MOZYME.
Acknowledgments
We thank the Ministerio de Educacion y Cultura for a Postdoctoral Fellowship (to C.C.).