| [Related articles/posters: 037 027 086 ] |
Introduction
The technique of Langmuir-Blodgett (LB) films id based on the formation of the molecular monolayer on aqueous sub-phase by an organic molecule having an amphiphilic structure. The substance of this type can spread over the aqueous surface and form a film having features of a two-dimensional gas. The spread film can be compressed, forcing in this way, an orientation of the amphiphilic molecules which can be achieved due to the van der Waals attraction interaction between long hydrophobic chains. The orientation of the organic molecules in the monolayer can be utilized on macroscopic scale for the formation of the material having nonlinear optical properties (NLO). However, modeling molecules for LB technique which at the same time would have NLO properties, one has to compromise two features of the molecule: the ability of the monolayer formation and the presence of a chromophore group with a good NLO response.
Working on synthesis of new amphiphiles for LB applications, the authors obtained three homologous series of heteroaromatic sulfonamides, shown below
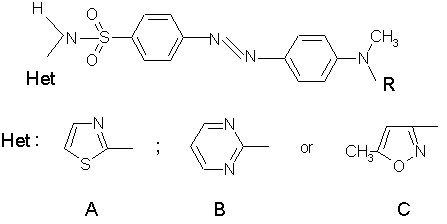
The hydrophobic fragment R has to be long enough to ensure the monolayer formation on aqueous sub-phase, and should contain more than 12 carbon atoms in an aliphatic hydrocarbon chain. For purposes of this study, the derivatives having n-hexadecyl chain were selected. The structure of the sulfonamides with electron donor-acceptor groups and double bonds conjugation via phenyl-azo-phenyl system is typical for NLO-phores with high first hyperpolarizability.
In this work we aimed to evaluate two features of the compounds shown in above figure:
Results and Discussion
Monolayer formation
The amphiphiles in question differ in structure of the heteroaromatic fragment: thiazolyl (A), pyrimidinyl (B) and methoxazolyl (C) while the rest of the molecule is the same. Fig.1 shows the compression isotherms of the amphiphiles determined at the room temperature (293 K).
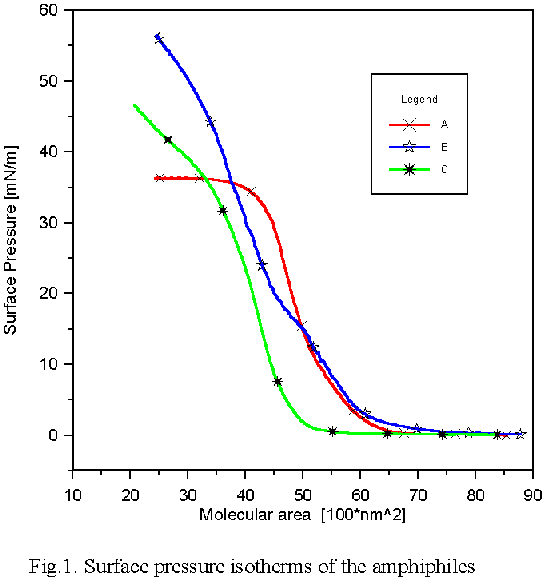
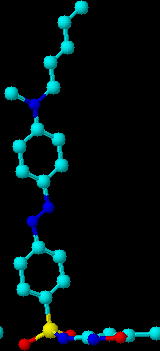
The derivative of sulfathiazole (A) shows a classical shape of the surface pressure isotherm with a break of the monolayer at ca. 36 mN/m. The run of the isotherm of sulfadiazine derivative (B) is similar to that of (A) at the lower range of surface pressure and then it runs its own way with no sharp break sign. The isotherm of the sulfomethoxazole derivative (C) is shifted to the lower molecular surface area , but the solid film molecular area is still higher than 40 nm^2 *100, i.e., twice the cross section area of the aliphatic chain. The formation of the monolayer film by these compounds is a sign that not only the amphiphiles of the pencil-like structure are suitable for monolayer formation. The quantum chemical optimized structures of the amphiphiles (A,B and C) for gaseous molecular state, presented below, clearly showed that heterocyclic ring was situated beyond the plane of phenyl-azo-phenyl fragment. The compounds (A) and (B) have similar structures while the compound (C) has a shape of its molecule similar to the letter L . We are, at this moment, not able to find whether the structures of the molecules at aqueous interface
are the same as those calculated for gaseous state, but what is obvious is a fact that even these spacially ătwistedämolecules can form oriented monolayer films transferable onto a solid sub-phase.

A
Fig.2. The molecular structures optimized by GAUSSIAN 3-21g option. Alkyl chains contain only 5 C atoms to keep pictures more compact.
B
First Hyperpolarizability
The nonlinear optical response of an isolated molecule in an electric field Ei (ω) can be presented as a Taylor series expansion of the total dipole moment, μt, induced by thefield,
where α is linear polarizability, μ
o is permanent dipole moment, and βijk are the first hyperpolarizability tensor components. The NLO response of the material in molecular state can be determined by computation and by measuring it experimentally. As the values obtained by different methods may be different, it is sometimes necessary to give an exact definition. In this paper we will consider only the frequency doubling process, i.e., β = β (-2ω,ω,ω ), and define in a molecule fixed coordinates
where i = x, y, or z
For calculation of the first hyperpolarizability by quantum chemical methods the GAUSSIAN and GAMESS programs were chosen at RHF (restricted Hartree-Fock) ab initio level of theory with a split valence 3-21g basis set.
The first hyperpolarizabilities, β, of the compounds in question are shown in Table.1. The values of static β are rather high and are comparable with that of 4-(dimethylamino)-4â-nitrostilbene. The lowest β value is attributed to the compound © whereas th
e amphiphiles (A) and (B) have comparable first hyperpolarizability values. If p-nitroaniline (PNA)could be used as a standard for NLO response then one obtains a relative measure of molecule hyperpolarizability. For our amphiphiles the β values are in the range of 6.26 to 7.11 that of PNA, if values obtained by GAMESS are taken into account. The β values obtained by GAUSSIAN CPHF calculation are higher by ca. 10 % than those calculates by GAMESS TDHF procedure.In this work we also used INDO1/S program with amphiphile structures (atomic coordinates) optimized by GAUSSIAN 3-21g runs, the same as mentioned above. We assumed the singlet state configuration interaction, and the space for calculation was HOMO-40 to LUMO+40 that produced 1600 configurations. Twenty electronic states were generated and the state S1 (the first excited state with oscillator strength f >0) was a dominant state responsible for the Π - Π* charge transfer process. The parameters of the state S1 and So were used to determine the charge transfer first hyperpolarizability, βCT , in a two-level model according to Equation (4).
where ,μ
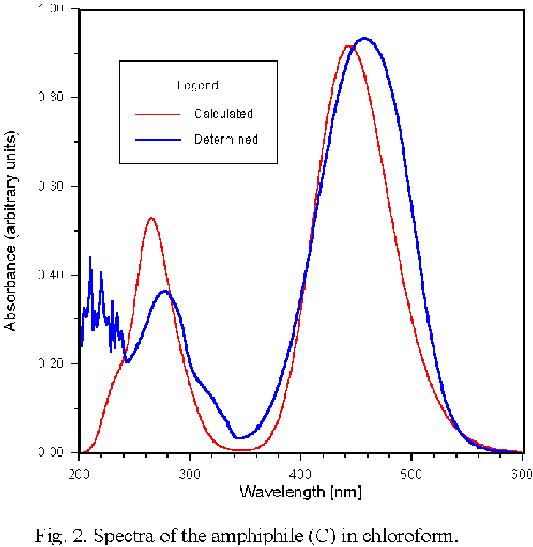
e, μg is a dipole moment in excited and ground state, respectively, μt is the transition moment between the excited and ground state, εo is vacuum permitivity, h is Planck constant and vt is the transition frequency from ground state to first excited state.The INDO1/S program is predestined to reproduce spectroscopic response of the molecule in isolated state and in the solution. In Fig.2 the determined UV-Vis spectrum of the compound © is confronted with that calculated by this program as an SCRF option in chloroform. The difference of the peak maximum of the lower energetic band ascribed to Π-Π* transition between calculated and determined wavelengths is small, and the same situation was observed in the case of compounds (A) and (B).
There are small discrepancies between values of ‰ obtained by Equation 4 and those calculated by ab initio procedures. In absolute scale, one may say that the first hyperpolarizability values (static) obtained by INDO1/S and GAMESS for the amphiphiles are of the same range of magnitude. The ‰ values of nitro derivatives are in this case overestimated, e.g., for PNA by ca 100% due to overestimation of the excited state dipole moment (Table 2)
TABLE 1: Dipole moments and first hyperpolarizability calculated by GAMESS and GAUSSIAN (G94) with 3-21g basis set (RHF).
|
Structure |
R2 |
GAMESS |
G94 |
|||
|
μ g *)
|
β o **) |
β 1064 **) |
β o/βoPNA |
β o **) |
||
|
A |
n-C16H33 |
38.89 |
215.6 |
339.5 |
7.11 |
241.1 |
|
B |
n-C16H33 |
32.12 |
212.4 |
333.7 |
7.00 |
232.1 |
|
C |
n-C16H33 |
35.10 |
189.6 |
304.6 |
6.26 |
198.0 |
|
p-nitroaniline (PNA) |
25.81 |
30.3 |
38.8 |
1.0 |
30.1 |
|
|
DANS***) |
32.20 |
180.3 |
|
5.95 |
212.2 |
|
*) dipole moments in [C*m]/10-30; **) values of β in [m4/V]/10-40 ;
***) DANS, 4-(dimethylamino)-4â-nitrostilbene
TABLE 2: Dipole moments and first hyperpolarizability calculated by INDO1/S (two-level model), structure optimization by Gaussian 3-21g.
|
Structure |
R |
Solvent |
μ g *) |
μ e *) |
μ t *) |
v m-1/106 |
β o *) |
|
A |
n-C16H33 |
none |
41.80 |
71.90 |
30.55 |
2.4613 |
197.8 |
|
chloroform |
45.13 |
78.21 |
30.83 |
2.3040 |
252.7 |
||
|
B |
n-C16H33 |
none |
36.30 |
64.01 |
30.51 |
2.4780 |
179.2 |
|
chloroform |
39.25 |
69.91 |
30.70 |
2.3270 |
227.7 |
||
|
C |
n-C16H33 |
none |
37.23 |
69.55 |
29.95 |
2.4589 |
204.6 |
|
chloroform |
40.89 |
76.70 |
30.17 |
2.2528 |
274.1 |
||
|
p-nitroaniline (PNA) |
none |
28.55 |
63.76 |
20.78 |
3.1952 |
63.6 |
|
|
DANS **) |
none |
30.06 |
82.04 |
31.07 |
2.5757 |
323.2 |
|
1. The amphiphiles of the type (A), (B) and (C) having n-hexadecyl aliphatic chain as a hydrophobe are able to form monolayer films on aqueous sub-phase. The monolayer film can be transferred onto a solid support giving thus an oriented molecular structure which can be made a multilayer one by a repeated deposition.
2. The amphiphiles in question showed NLO response expressed in terms of the first hyperpolarizability which may be compared with that of azo derivatives of p-nitroaniline and 4-(dimethylamino)-4=-nitrostilbene (DANS).
Appendix
4-(4=-N-methyl-N-n-hexadecylaminophenylazo)-N-(thiazol-2-yl)benzenesulfonamide (A)
Yield: 84 %; m.p.: 174-175oC; 1H NMR (CDCl3, TMS): 0.88, t, 3H (CH3C); 1.31, m, 26H (CH3(CH2)13CH2CH2N); 1.63, m, 2H (CH3(CH2)13CH2CH2N); 3.06, s, 3H (CH3N); 3.41, t, 2H (CH3(CH2)13CH2CH2N); 6.52, d, J=4.6 Hz, 1H (C=CH-S of thiazole ring); 6.71, d, 2H (ortho protons vs. NCH3R of phenyl ring); 7.17, d, J=4.6 Hz, 1H (C=CH-N of thiazole ring); 7.86, d, 4H (ortho protons vs. -N=N- group of phenyl rings); 8.00, d, 2H (ortho protons vs. NHSO2 group of phenyl ring); 12.93, br. s, 1H (NHSO2).
4-(4=-N-methyl-N-n-hexadecylaminophenylazo)-N-(pyrimidin-2-yl)benzenesulfonamide (B)
Yield: 60 %; m.p.: 154.5-156oC; 1H NMR (CDCl3, TMS): 0.88, t, 3H (CH3C); 1.31, m, 26H (CH3(CH2)13CH2CH2N); 1.62, m, 2H (CH3(CH2)13CH2CH2N); 3.06, s, 3H (CH3N); 3.41, t, 2H (CH3(CH2)13CH2CH2N); 6.71, d, 2H (ortho protons vs. NCH3R of phenyl ring); 6.98, t, J=4.7 Hz, 1H (C=CH-C of pyrimidine ring); 7.87, dd, 4H (ortho protons vs. -N=N- group of phenyl rings); 8.20, d, 2H (ortho protons vs. NHSO2 group of phenyl ring); 8.67, d, J=4.7 Hz, 2H (N-CH=C-CH-N of pyrimidine ring); 11.88, br. s, 1H (NHSO2).
4-(4=-N-methyl-N-n-hexadecylaminophenylazo)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (C)
Yield: 76 %; m.p.: 108.5-110.5oC; 1H NMR (CDCl3, TMS): 0.88, t, 3H (CH3C); 1.27, m, 26H (CH3(CH2)13CH2CH2N); 1.64, m, 2H (CH3(CH2)13CH2CH2N); 2.37, s, 3H (CH3 group of 5-methylisoxazole ring); 3.07, s, 3H (CH3N); 3.42, t, 2H (CH3(CH2)13CH2CH2N); 6.26, s, 1H (C=CH-C=N of 5-methylisoxazole ring); 6.71, d, 2H (ortho protons vs. NCH3R of phenyl ring); 7.86, d, 4H (ortho protons vs. -N=N- group of phenyl rings); 7.92, d, 2H (ortho protons vs. NHSO2 group of phenyl ring); ~ 9.0, v. br. s, 1H (NHSO2).
References
1. Kucharski S.; Janik R. Bull. Polish. Acad. Sci. (Chem.), 1997, 45(3),
2. Kucharski S., Janik.; Kaatz P. J.Phys.Chem., 1997, 101(44B), 8967
3. Ulman A.; Willand C.S.; Kšhler W.; Robello D.R.; Williams D.J.; Handley L. J.Am.Chem Soc. 1990, 112 , 7083
4. Le Breton H.; Letard J-F.; Lapouyade R.; Le Calvez A.; Maleck Rassoul R.; Freysz E.; Ducasse A.; Belin C.; Morand J-P. Chem.Phys.Lett. 1995, 242, 604
5. van Hutten P.F.; Hadzioannou G.; Bursi R.; Feil D. J.Phys.Chem. 1996, 100, 85