| [Related articles/posters: 037 122 086 ] |
Purpose: Synthesis of N-sulfonyl 2-diazo malonamide derivatives and study of their intramolecular cyclizations to N-arylsulfonyl-1,2,3-triazole derivatives are described
Introduction
The chemistry of aliphatic diazo compounds is intensively developing area of organic chemistry. In this class diazo acetamides are of the special interest. On their basis, the synthesis of 1,2,3-triazoles and -thiadiazoles possessing the biological activity is carried out. At the same time reactivity of N-sulfonyl diazo acetamides was not investigated so far, that is necessary to develop the new methods for the synthesis of azoles.
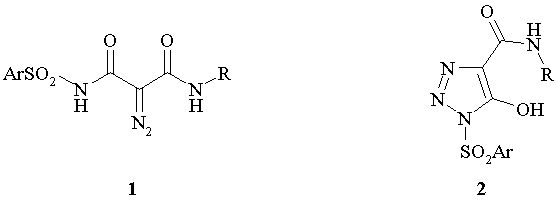
In the present paper, we describe the synthesis N-sulfonyl diazo acetamides 1 and reactions of their intramolecular cyclization to 1-sulfonyl 1,2,3-triazoles derivatives 2.
Results and Discussion
The synthesis of N-sulfonyl diazo acetamides 1 and isomeric 1-sulfonyl 1,2,3-triazoles derivatives 2 .was implemented as four-step process where sulfonyl amides 3 were used as starting material. Treatment of compounds 3 with aqueous alkali lead to acids 4 which in subsequent reaction with amines in the presence of CDI gave amides 5. The resulting amides were subjected to a diazotransfer reaction with tosyl azide and two moles of sodium ethylate in ethanol to form sodium triazolates 6a.
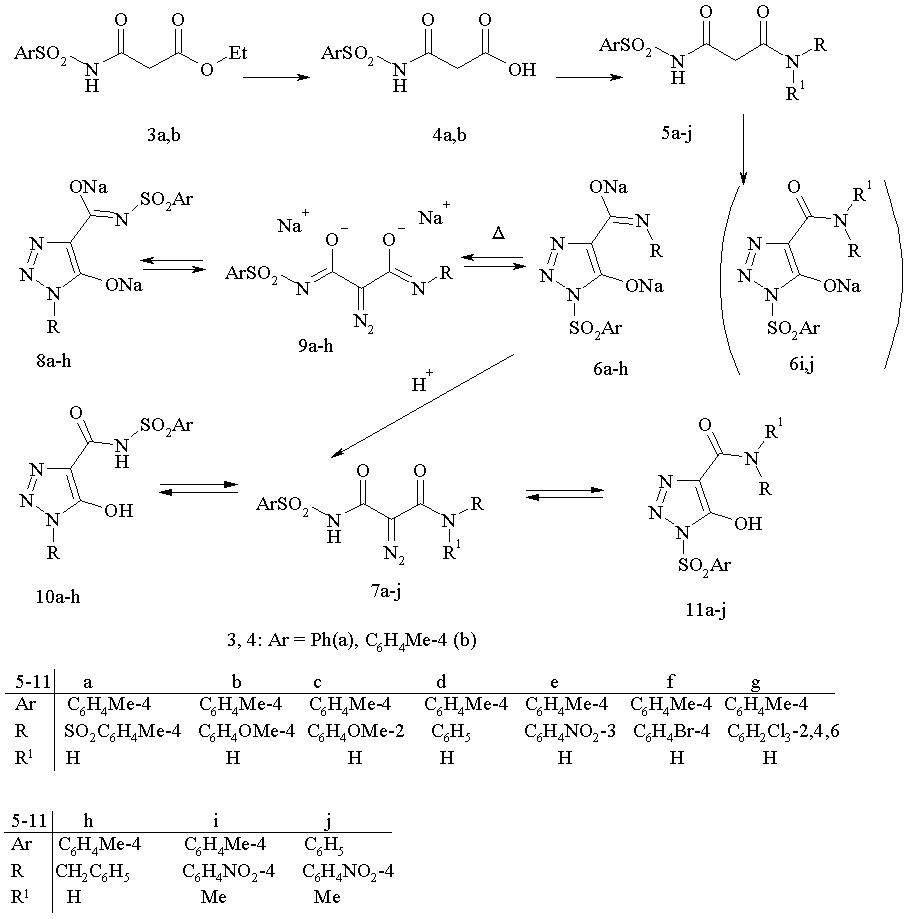
In 1H NMR spectrum for 6a the signals of aromatic protons were observed as four doublets at 7.85, 7.70, 7.29 and 7.22 ppm; two three-protons singlets at 2.364 and 2.324 ppm, which were referred to protons of tosyl moiety in the position 1 and to protons of carbamoyl group, respectively. Work up of water solution of triazoles 6a with 1N the hydrochloric acid gave a product. In spectrum NMR 1H of this product two two-protons doublets of aromatic protons were observed at 7.70 and 7.21 ppm and one three-proton singlet at 2.32 ppm; IR spectrum of this product contains absorption band at 2120 cm-1 (diazo group). On the basis of these data the structure of N,N'-bistosyl diazo malone amide 9a was attributed to this compound.
Similarly the syntheses of triazoles 6i,j and diazo compounds 9i,j, bearing two substituents at the nitrogen atom of carbamoyl function were performed. In the spectrum 1H NMR of 1-tosyl-1,2,3-triazole 6i a singlet of methyl group at 2.36 ppm is observed, and for diazo compound 9i - at 2,34 ppm. In the IR spectra of compounds 9i,j the absorption band of diazo group was observed at 2120 cm-1.
In a diazo transfer reaction of asymmetrically substituted malone amides 5b-h, more complex pattern is expected: two direction for the cyclization of diazo function either onto tosylcarbamoyl or to arylcarbamoyl to afford isomeric triazoles 6b-h and 8b-h may be achieved. Actually diazo group transfer reaction with 5b-h lead solely to individual 1-tosyltriazoles 6b-h. For derivatives 8b,c in a spectrum 1H NMR a low field shift of signals of protons of methoxy group to 3.73 ppm (d 0.11 as compared with triazoles 6b,c) is observed. Heating of compounds 6b-h in water lead to rearrangement products 8b-h. 1H NMR spectra of these compounds contain the signals of methyl group of tosyl moieyty at 2.318 ppm; for compounds 8b,c as compared with triazoles 6b,c a low field shift for the signals of protons of methoxy groups (3.84 ppm) for 0.12 ppm was observed; in IR spectra absorption band, characteristic for diazo group, is not observed. On the basis of these data the structure 1-aryl-1,2,3-triazoles 8b-h, with tosyl group in carbamoyl function at position 4 of the ring was assigned to these compounds.
Exposure for a long time of tosyl triazoles 6a-h in a solution DMSO-D6 or in CDCl3 in tube of NMR spectrometer displayed one or more additional set of signals, which were referred to aryltriazoles 8d-h and diazo malonamides 9a-h. The signals of protons for methyl of tosyl function and for methoxy group (for 9b,c) were observed at 2.321 ppm and at 3.76-3.75 ppm respectively. In IR spectrum for this inseparable mixture of three compounds an absorption band of diazo group at 2130 –“-1 was registered. The similar data were obtained for isomers 8. The data on the composition of equilibrium mixtures of compounds 6,8,9 determined from integral signals for protons of methyl group and aromatic ring are given in table 1. It is worth noting that the composition of mixtures of compounds obtained from both triazoles 6 and 8 coincided within the limits of an error of measurement (2-5 %).
Table 1
Ratio of isomers 6,8,9 in solution of DMSO-D6
|
Compound |
Ratio, % of isomers |
|||
|
R in Ar |
6 |
8 |
9 |
|
|
b |
p-OMe |
16 |
42 |
42 |
|
c |
o-OMe |
42 |
<1 |
58 |
|
d |
H |
30 |
35 |
35 |
|
f |
p-Br |
35 |
25 |
40 |
|
g |
2,4,6-Cl3 |
10 |
<1 |
90 |
|
e |
m-NO2 |
<1 |
>99 |
<1 |
An attempt to isolate diazo compounds 7a-j from water solutions of triazoles 6a-j by treatment with hydrochloric acid lead to individual compound (TLC), which IR spectrum displayed the band of diazo group at 2120 –“-1. However, according data of NMR it exists in DMSO-D6 or CDCl3 as a mixture of 1-sulfonyl triazoles 10 and 1-aryl triazole 11.
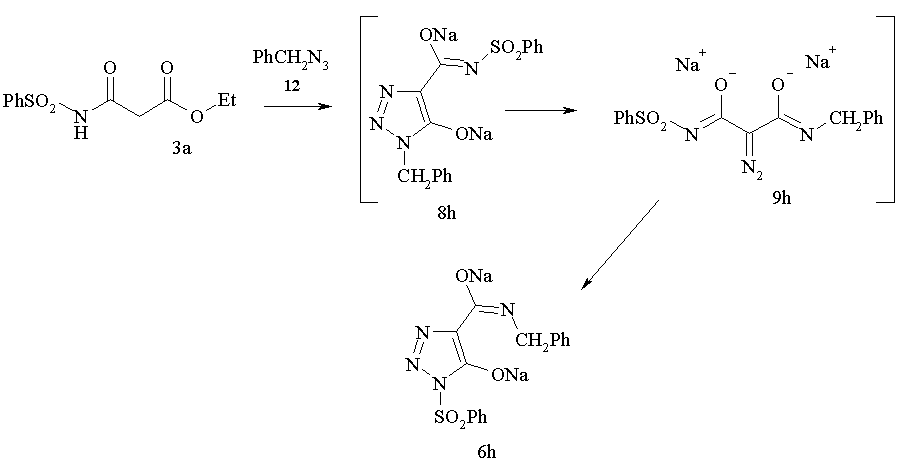
It is intersting to note that diazo group transfer reaction onto malonamide 3a with benzyl azide accompanied with the rearrangement of initially formed N-benzyl 1,2,3-triazol5-olate 8h to afford N-sulfonyl 1,2,3-triazol 6h in good yield.
Conclusions:
We have found that 1-tosyl-1,2,3-triazole-4-N-arylcarbanoyl-5-olates can rearrange to isomeric 1-aryl-1,2,3-triazole-4-N-sulfonylcarbamoyl-5-olates. The equilibrium between these compounds and transient diazo compound has been shown to exist in the solutions of DMSO and in chloroform. The introduction of electron-withdrawing substituents in aryl group rings stabilizes that isomeric heterocycle which has aryl moiety at the position 1 of the ring. We have found that in contrast to other substituents, ortho-substituents in aryl stabilize the chain isomer, diazo compounds.
Acknowledgments
We gratefully acknowledge the Russian Foundation for Basic Research (Grant 98-03-33045a) for financial support of this work.
Experimental section
IR spectra were registered on a Specord IR 75 spectrometer in KBr pellets (Table 5). The 1H and 13C NMR spectra were recorded on Bruker 250 (250 MHz) spectrometer in DMSO-D6 and CDCl3 with TMS as internal reference. All the reaction mixtures and products obtained were analyzed by TLC on Silufol UV-254. The results of microanalysis (C,H,N,S) for the compounds prepared were as required.
General procedure to prepare 1-sulfonyl 1,2,3-triazoles (6a-j)
1.83 mG (10 mMol) of tosyl azide was added dropwise to the stirred solution of 20 mMol of sodium ethylate and 10 mMol of a malonamide 4a-j in 10 mL of ethanol at room temperature. The reaction mixture was stirred for 3 h and the crude precipitate was filtrated, washed with ether and dried.
General procedure for 4-N-sulfonylcarbamoyl 1,2,3-triazole (8a-h)
1.83 mG (10 mMol) of tosyl azide was added dropwise to the stirred solution of 20 mMol of sodium ethoxide and 10 mMol of a malonamide 4a-j in 10 mL of ethanol at room temperature. The reaction mixture was stirred for 3 h and the crude precipitate was filtrated, crystallized from ethanol and dried.
General procedure for diazo compounds (9a-h)
1.83 mG (10 mMol) of tosyl azide was added dropwise to the stirred solution of 20 mMol of sodium ethoxide and 10 mMol of a malonamide 4a-j in 10 mL of ethanol at room temperature. The reaction mixture was stirred for 3 h and the solvent was removed. 10 mL water was added and the crude precipitate was filtrated. The filtrate was concentrated in vacuo at 70oC, the residue was treated with 30 mL of dry ether and filtered to give diazo compounds 9.
Table 2
NMR 1H spectra of compounds 6a-j, 8b,d-h, 9a,c,i,j
|
Compound |
NMR 1H spectra |
|
|
6a |
7.85 (2H, d, ArH), 7.70 (2H, d, ArH),7.29 (2H, d, ArH),7.22 (2H, d, ArH),2.364 (3H, s, ArCH3), 2.324 (3H, s, ArCH3) |
|
|
9a |
7.70 (2H, d, ArH),7.21 (2H, d, ArH), 2.316 (3H, s, ArCH3), |
|
|
6b |
7.82 (2H, d, ArH), 7.68 (2H, d, ArH), 7.42 (2H, d, ArH), 6.85 (2H, d, ArH), 3.73 (3H, s, ArOCH3), 2.360 (3H, S, ArCH3) |
|
|
8b |
7.84 (2H, d, ArH), 7.63 (2H, d, ArH), 7.36 (2H, d, ArH), 6.98 (2H, d, ArH), 3.84 (3H, s, ArOCH3), 2.318 (3H, S, ArCH3) |
|
|
6c |
8.27 (1H, ddd, ArH), 7.87 (2H, d, ArH), 7.40 (2H, d, ArH), 7.18 (1H, ddd, ArH), 7.14 (1H, ddd, ArH), 7.02 (1H, ddd, ArH), 3.72 (3H, s, ArOCH3), 2.319 (3H, s, ArCH3) |
|
|
8c |
8.30 (1H, ddd, ArH), 7.84 (2H, d, ArH), 7.38 (2H, d, ArH), 7.18 (1H, ddd, ArH), 7.14 (1H, ddd, ArH), 7.02 (1H, ddd, ArH), 3.86 (3H, s, ArOCH3), 2.390 (3H, s, ArCH3) |
|
|
9c |
8.28 (1H, ddd, ArH), 7.84 (2H, d, ArH), 7.38 (2H, d, ArH), 7.18 (1H, ddd, ArH), 7.14 (1H, ddd, ArH), 7.02 (1H, ddd, ArH), 3.70 (3H, s, ArOCH3), 2.320 (3H, s, ArCH3) |
|
|
6d |
7.60-7.70 (2H, m, Ph), 7.30-7.40 (3H, m, Ph), 7.38 (2H, s, ArH), 7.14 (2H, d, ArH), 2.215 (3H, s, ArCH3) |
|
|
8d |
7.58-7.65 (2H, m, Ph), 7.30-7.40 (3H, m, Ph), 7.34 (2H, d, ArH), 7.15 (2H, d, ArH), 2.275 (3H, s, ArCH3) |
|
|
6e |
9.15 (1H, dd, ArH), 8.49 (1H, ddd, ArH), 8.01 (1H, ddd, ArH), 7.78 (2H, d, ArH), 7.70 (1H, dd, ArH), 7.20 (2H, d, ArH), 2.390 (3H, s, ArCH3) |
|
|
8e |
9.15 (1H, dd, ArH), 8.48 (1H, ddd, ArH), 8.02 (1H, ddd, ArH), 7.79 (2H, d, ArH), 7.70 (1H, dd, ArH), 7.20 (2H, d, ArH), 2.318 (3H, s, ArCH3) |
|
|
6f |
8.06 (2H, d, ArH), 7.58 (2H, d, ArH), 7.79 (2H, d, ArH), 7.16 (2H, d, ArH), 2.360 (3H, s, ArCH3) |
|
|
8f |
8.07 (2H, d, ArH), 7.57 (2H, d, ArH), 7.78 (2H, d, ArH), 7.18 (2H, d, ArH), 2.318 (3H, s, ArCH3) |
|
|
6g |
7.83 (2H, d, ArH), 7.70 (2H, s, ArH), 7.28 (2H, d, ArH), 2.354 (3H, s, ArCH3) |
|
|
8g |
7.67 (2H, s, ArH), 7.64 (2H, d, ArH), 7.16 (2H, d, ArH), 2.316 (3H, s, ArCH3) |
|
|
9g |
7.68 (2H, d, ArH), 7.58 (2H, s, ArH), 7.21 (2H, d, ArH), 2.325 (3H, s, ArCH3) |
|
|
6h |
7.85 (2H, d, ArH), 7.40 (5H, s, Ph), 7.29 (2H, d, ArH), 5.36 (2H, s, PhCH2), 2.324 (3H, s, ArCH3) |
|
|
8h |
7.70 (2H, d, ArH), 7.55 (5H, s, Ph), 7.21 (2H, d, ArH), 5.60 (2H, s, PhCH2), 2.316 (3H, s, ArCH3) |
|
|
6i |
8.08 (2H, d, ArH), 7.67 (2H, d, ArH), 7.53 (2H, d, ArH), 7.23 (2H, d, ArH), 3.22 (3H, s, NCH3), 2.36 (3H, s, ArCH3) |
|
|
9i |
8.01 (2H, d, ArH), 7.60 (2H, d, ArH), 7.47 (2H, d, ArH), 7.20 (2H, d, ArH), 3.22 (3H, s, NCH3), 2.33 (3H, s, ArCH3) |
|
|
6j |
8.08 (2H, d, ArH), 7.60-7.70 (2H, m, Ph), 7.54 (2H, d, ArH), 7.30-7.40 (3H, m, Ph), 3.215 (3H, s, NCH3) |
|
|
9j |
8.00 (2H, d, ArH), 7.58-7.65 (2H, m, Ph), 7.50 (2H, d, ArH), 7.30-7.40 (3H, m, Ph), 3.215 (3H, s, NCH3) |
|