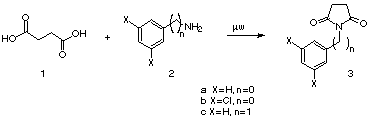Synthesis of 2,5-Pyrrolidinediones by Microwave Heating. A Direct Entry to Fungicides.
Julio A. Seijas*, M. Pilar Vázquez-Tato*, M. Montserrat Martínez
and Gonzalo Núñez
Departamento de Química Orgánica. Universidad de Santiago
de Compostela. Facultad de Ciencias de Lugo. Aptdo. 280. 27080-Lugo. Spain.
Imide derivatives are associated with various uses both by its biological[1]
or industrial[2]
properties. Our interest was specially directed towards 2,5-pyrrolidinediones
since among them there are fungicides of agricultural use[3],
Our method consists in heating the acid mixed with the amine in a molar
ratio 1:1 up to the disappearance of the starting material. Thus, succinic
acid (1) was heated with aniline (2a) yielding N-phenylsuccinimide
(3a) in 76%; when the amine employed was 2,5-dichloroaniline (2b)
the reaction afforded fungicide 3b in 78% yield, and with benzylamine
(2c) we obtained a similar yield (78%) of compound 3c.
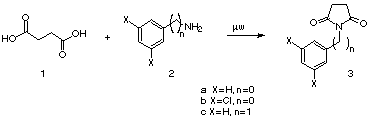
We compared these reaction conditions with conventional heating, and we
found that reaction times were shorter and yields higher with microwaves.
Click
here for full poster
[1]
Wang, J.J., Wang, S. S., Lee, C. f., Chung, M. a. Chern, Y. t., Chemotherapy
(1997), 43, 182-189. Da settimo, A., Primofiore, G., Da Settimo,
F.; Simorini., F.; La Motta, C.; Martinelli, A.; Boldrini, E.; Eur.
J. Med. chem. (1996), 31, 49.;
[2]Umeki,
H., Ogawa, T., Chem. Abstracts, 115, 234443s. Scherowsky,
G., Schreiber, P., Cham. Abtracts, 113, 241602e.
[3]Fujinami,
A., Ozaki, T., and Yamamoto, S., Agric. Biol. Chem., (1971). 35,
1707. Ozaki, t., fujinami, S., Yamamoto, T. Katsutoshi, T., Oishi, T. and
Nodera, K., Chem. Abstracts. (1974), 80, 56455w.