|
Synthesis of 2,5-Pyrrolidinediones by Microwave Heating. A Direct Entry to Fungicides |
Julio A. Seijas*, M. Pilar Vázquez-Tato*, M. Montserrat Martínez and Gonzalo Núñez
Departamento de Química Orgánica. Universidad de Santiago de Compostela. Facultad de Ciencias de Lugo. Aptdo. 280. 27080-Lugo. Spain.
In the field of synthetic organic chemistry, the development of simple general synthetic routes from readily available reagents is one of the major challenges. In the last years the use of microwaves to "emulate" classic organic reactions has become very popular amongst synthetic organic chemists, because when the microwave heating is applicable, it leads to higher yields, cleaner reactions and shorter reaction times.
Imide derivatives are associated with various uses both by its biological [1] or industrial [2] properties. Our group's interest was specially directed towards 2,5-pyrrolidinediones since among them there are fungicides of agricultural use. One of us already had used microwaves to prepare amides by pyrolisis of carboxylic acid salts of amines [3]. We thought that this could be extended to the synthesis of imides. The previously described methods employ mainly N-alkylation of imides [4] and reaction of dicarboxylic acids or its anhydrides with amines heating at high temperature (aproximately in a range between 150-200°C, and reaction times usually longer than 30 minutes) [5].
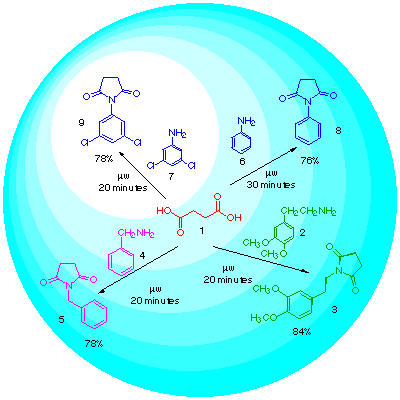
Our method consists in heating in a domestic microwave oven the acid mixed with the amine in a molar ratio 1:1 up to the disappearance of the starting material.We tried the reaction with succinic acid and several amines: aliphatic and anilines. Thus, succinic acid (1) was heated with homoveratrylamine (2) during 20 minutes yielding N-phenethylsuccinimide (3) in 84%; when benzylamine (4) was used we obtained N-benzylsuccinimide (5) in a 78% yield, and with anilines (6) and (7) we obtained N-phenylsuccinimide (8) and the known fungicide 9 in 76% and 78% yield respectively.
Recently a paper appeared where is used microwave irradiation to prepare imides from anhydrides and in the presence of TaCl5-SiO2 [6], we think our method is an improvement of this one since we avoid the use of the anhydride, sometimes not easily affordable, and the use of heavy metals, which is really a matter of environmental concern when trying to extend a method to a multigram scale synthesis.

Acknowledgements: We thank to DGES(Spain) for financial support PB96-0932
1.Wang, J.J.; Wang, S.S.; Lee, C.F.; Chung, M.A.and Chern, Y.T.; Chemotherapy (1997), 43, 182-189. Da Settimo, A.; Primofiore, G.; Da Settimo, F.; Simorini, F.; Martinelli A. and Boldrini, E.; Eur. J. Med. Chem., (1996), 31, 49.
2.Umeki, U.; Ogawa, T.; Chem. Abstracts, 115, 234443s. Scherowsky, g.; Schreiber, P.; Chem. Abstracts, 113, 241602e.
3. Vázquez-Tato, M.P.; Synlett, (1993), 506.
4. Walker, M. A.; J. Org. Chem.; (1995), 60, 5352.
5. Hargreaves, M. K.; Pritchard, J. G.; Dave, H.R.; Chem. Rev.; (1970), 70, 439. Hadley, M.S.; King, F.D.; Martin, R.T.; Tetrahedron Lett., (1983), 24, 91. Fadel, A.; García-Argote, S.; Tetrahedron Asimm.; (1996), 7, 1159.
6. Chandrasekhar, S., Takhi, M; Uma, G.; Tetrahedron Lett. (1997), 46, 8089.