| [Related articles/posters: 055 125 009 ] |
Previously [1,2] some factors governing the direction of the reaction of trichloromethylarenes (TCMA) with acylhydrazines and thioacylhydrazines were studied. In alcohols as solvents benzotrichloride (1a) and its substituted derivatives give predominantly alkyl arenecarboxylates formed as the result of alcoholysis while target 1,3,4-oxadiazoles (thiadiazoles) are minor products. In pyridine solutions the products of reductive condensation, viz. respective N-substituted aromatic aldohydrazones are formed preferentially or exclusively. For heterocyclization leading to 1,3,4-oxadiazole (2) of thiadiazole derivatives to carry out the reaction in a mixture of pyridine with methanol or ethanol appeared to be optimal conditions. At the same time in reactions of TCMA with an equimolar amount of hydrazine hydrate in a pyridine - methanol mixture, symmetrically substituted 2,5-diaryl-1,3,4-oxadiazoles were obtained in low yields (~20%) and besides methyl esters and hydrazides of respective arenecarboxylic acids were isolated. Now we found out unexpectedly that the yield of 2,5-diphenyl-1,3,4-oxadiazole (2a) became near to quantitative when pyridine was excluded and the reaction was carried out on refluxing 40 min in ethanol using excess hydrazine as an HCl acceptor. 4-Chlorobenzotrichloride (1b) and 3-bromobenzotrichloride (1c) reacted with hydrazine similarly to benzotrichloride, yields of compounds 2b-c were 81 and 68%, respectively.
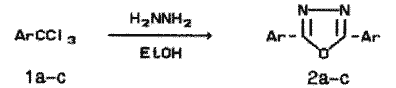
a Ar = Ph, b Ar = 4-ClC6H4, c Ar = 3-BrC6H4
Reactions of bis(trichloromethyl)benzene (3) with acylhydrazines (4) were shown to be very simple route (though yields are moderate) of 1,4-phenylene-bis-1,3,4-oxadiazoles (5), which, as is well known, manifest luminescent activity.

a R = Ph, b R = 2-HOC6H4, c R = 4-O2NC6H4, d R = 4-C5H4N, e R = H
1. I. S. Poddubnyi, L. I. Belen'kii, and M. M. Krayushkin, Khim. Geterotsikl. Soed., 1994, 686. [Chem. Heterocycl. Comp., 1994, 30, 602 (Engl. Transl.)].
2. I. S. Poddubnyi, L. I. Belen'kii, and M. M. Krayushkin, Izv. Akad. Nauk. Ser. Khim., 1996, 1246. [Russ. Chem. Bull., 1996, 45, 1185 (Engl. Transl.)].