| [Related articles/posters: 087 114 055 ] |
Readily available O-heterocycles
of the pyrylium, benzo[b]pyrylium or xanthylium type, together with their
thiopyrylium and pyridinium analogs, play important roles in synthetic,
physical-organic, and pharmaceutical chemistry, finding diverse high-tech
applications.[96CHEC469, 96CHEC245]
Pyrylium derivatives are important as absorption/fluorescent probes with
high quantum yields [89MI805, 92MI880,
93MI561] for use in ion detection,[96MI]
for DNA-sequencing,[95MI247] as laser
dyes [74MI81, 89MI13]
and as potential photochemical therapeutic agents. [90JA3845]
As avid electron acceptors pyrylium salts form pyranyl radicals and can
be used as initiators for radical reactions.[94CRV1063,
89BCJ2279] Benzo[b]pyrylium cations occur
in the chromophors of the natural anthocyanines, the color pigment of many
flowers, fruits and leaves, and are used as drug colorants.[83T3005]
Xanthylium dyes absorbing or emitting in the red and near infrared spectral
region (NIR) are of particular interest in chemical and biochemical sensing
among other uses.[96MI] Specifically designed
functional benzo[b]pyrylocyanines have found applications in sensor techniques.[93Pat,
94DP229, 96MI395]
Proton acceptor/donor functionality enable xanthylium derivatives to act
as pH sensitive dyes, in which the NIR absorption bands can be switched
on by protonation (Scheme 1).
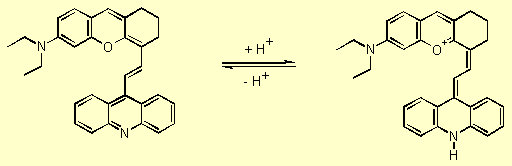
Although
a multitude of functional dyes have been described, only a small fraction
have found practical application. The technological applications for all
of the aforementioned intelligent dyes are often significantly restricted
by limited solubility, relatively high polarity, and absence of functionality
which selectively allows for the direct derivatization/immobilization.
Another disadvantage is the instability of many p-deficient
cations in solutions at pH > 7. Consequently, new variations are
needed, functionally tailored to meet the requirements of different
applications. The synthetic tools available for organic chemists to modify
heteroarylium derivatives are limited by the specific reactivity of these
p-deficient heterocycles, which generally allow
only nucleophilic substitution.[91HOU30]
To access new functional dyes, with defined physical and chemical properties,
requires new synthetic methodology.
Although
a few indirect electrophilic substitutions of heteroarylium salts have
previously been achieved [77DOK599, 85JPC983]
there were no general methods which allow overall electrophilic substitution
in the position 2 or 4 to the heteroatom prior to our recently introduced
methodology of derivatization of heterocycles 3 at the position
4 to the heteroatom, utilizing benzotriazole as a synthetic auxiliary.[91T2683,
94MI31, 98CRV409]
Benzotriazole,
like a chemical chameleon, plays a role in each of the four
steps of the substitution procedure. In step 1, it acts as a nucleophile
to give the corresponding 4H-(benzotriazol-1-yl)heterocycles 5.
The novel anion precursors 5 undergo smooth lithiation at the position
a to the benzotriazolyl moiety to form the benzotriazole
stabilized anions 6 (step 2). Subsequent trapping of 6 with
various electrophiles gives intermediates 7 of which several derivatives
were isolated and characterized. [97H2413,
98JOC3450]
2nd step:
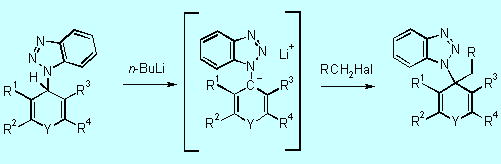
5 6 7
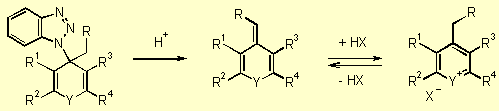
Finally,
benzotriazole acts as a good leaving group. After addition of a weak acid
the exomethylene-containing heterocycles 8 can be obtained (Scheme
4, step 3). Without isolation, intermediates 7
can be treated directly with strong mineral acids (usually perchloric acid)
to induce removal of benzotriazole and generate modified heteroarylium
salts 9 by protonation of base 8 (Scheme 4, step 4).
This new methodology allows the molecular tailoring of p-electron-deficient heterocycles with interesting properties and provides a convenient alternative and extention to the known methods. There are many readily available pyrylium, benzo[b]pyrylium, xanthylium, thioxanthylium, and N-methylacridinium systems, examples of all of which have been shown to undergo indirect electrophilic substitution via their benzotriazole substituted intermediates 5.[97JOC8198, 97H2413, 98uw] One series with interesting photophysical properties (e.g. shape and lmax of absorption bands, and fluorescence quantum yields, with respect to solvent polarity) is that containing the saturated 3,2'-bridged benzo[b]pyrylium nucleus.[98uw]
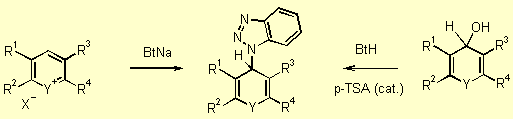
We have developed different pathways to the starting materials of type 5. (Figure 1, 43 Kb). The most efficient route to synthesize these materials is shown in Scheme 2, step 1. Various heteroarylium salts 3 react with sodium benzotriazolate to give the corresponding benzotriazole derivatives 5. [97JOC8198] The synthesis proceeds under mild conditions at room temperature. Nearly quantitative yields were obtained by using THF as solvent and NaH as base. 9H-Benzotriazolyxanthene and thioxanthene derivatives analogous to 5 were synthesized by the dehydration of the corresponding carbinol derivatives, 9H-10-oxaanthracen-9-ol and 9H-10-thioanthracen-9-ol of type 4 respectively, in presence of catalytic amounts of acid (Scheme 2). [98JOC3450] In all cases, mixtures of the benzotriazolyl-1 and benzotriazolyl-2 isomers 5 were obtained. For analytical purposes the individual isomers could be isolated easily from each pair by crystallization or by chromatography on silica gel. For the generation of carbanions 6 these isomeric mixtures can be used without separation. Compounds of type 5 are colorless, crystalline, and stable to neutral and basic conditions, but easily eliminate benzotriazole to regenerate the mostly colored, highly fluorescent salts 3 upon treatment with even weak acids.
The electron-withdrawing benzotriazole moiety in compounds 5 has powerful ability to promote proton abstraction from the a-carbon atom. Conversion of 5 to the carbanion 6, which is sufficiently stabilized to allow reaction with electrophiles, proceeds at low temperature (-78 ºC) in dry THF with n-butyllithium. A variety of electrophilic systems can be applied to molecularly engineer p-deficient heterocycles using benzotriazole methodology. Use of alkyl halides gives easy access to alkyl substituted heteroarylium derivatives, whereas the introduced p-nonconjugated residue does not significantly affect the photophysical properties of this familiar p-skeleton.[89MI805] Varying the chain length of the electrophiles, we synthesized as examples 4-methyl-, 4-(n-butyl)-, 4-(n-dodecyl)-, and 4-(n-octadecyl)heteronium salts and extended the method to alkyl chains of 22 carbons [97JOC8198, 97H2413] by utilizing C22H45Br, which is among the longest of the commercially available carbon-chain electrophiles. The products are crystalline materials isolated simply by recrystallization of the crude reaction mixtures in essentially quantitative yields.
In contrast to the starting heterocycles 3, the new salts 9 are noticeably acidic due to the protons of the alkyl substituents in position a to the p-deficient heteroaromatic cations. As a result, treatment of salts 9 with a solution of an inorganic base allows the isolation of the free bases 8. (Figure 2, 15 Kb) [97H2413, 98JOC3450] Significantly, heterocycles of type 8 and 9 with a long chain alkyl group in the position 4 to the heteroatom of the heterocycle were not previously described. However, Balaban et al. have recently reported the synthesis of pyrylium salts with long alkyl chains in the 2- and 6-positions by standard methods.[96RRC979, 92ZN1011]
Reactions of the heteronium anions 6 with bifunctional electrophiles such as a,w-dihalogenoalkanes was also successful.[97H2413, 98JPCip, 98uw] When the ratio of electrophile (1,4-diiodobutane or 1,10-dibromodecane) to anion 6 is one to two, the a,w-bis-heteronium derivatives (Figure 3, 25 Kb) were conveniently prepared in high yield. By using an excess of 1,10-dibromodecane as electrophile the mono-addition products were prepared, but in considerably lower yield, sometimes along with the previously mentioned bis-derivatives as a by product. However, 1,4-diiodobutane reacts smoothly with some heteronium anions to give mono-addition product only, even in attempts to synthesise the 1,4-bis-heteronium derivatives.[97H2413] The synthesis of mono-addition products could be facilitated by using dihalogenated electrophiles with different halogens. Thus, the reaction of the anions 6 generated in situ with one equivalent of 1-bromo-3-chloropropane or of 1-bromo-4-chlorobutane gives the mono-addition products 9. (Figure 4, 20 Kb). These compounds have potential for further derivatization, and could be used for the synthesis of unsymmetrical bifunctional derivatives (e.g. bichromophores).
We also employed benzyl bromide, 1,4-bis-(bromomethyl)benzene and longer w-aryl-substituted alkyl bromides (w-bromoethylbenzene and 1-bromo-3-phenylpropane) successfully as electrophiles. The protons of the benzyl substituents in the position a to the heteroaromatic cations are significantly acidic. This behavior can be used to design useful stable acidochromic dyes (Figure 5, 18 Kb).[98uw-2]
Benzotriazole
mediated electrophilic substitution of heteroaromatic systems 3
also allows the synthesis of analogs of compound 1 as new monomethinecyanine
precursors. Starting from readily available and well characterized 4H-(1H-benzotriazol-1-yl)-benzo[b]pyrans
10 and 9-(a-bromomethyl)acridine as electrophile,
we obtained acidochromic monomethine precursors 11. In contrast
to compounds 8/9, protonation of 11 is observed on the nitrogen
of the heterocycle generating the deeply colored
monomethinecyanine dye 12 (Figure 6, 16 Kb).
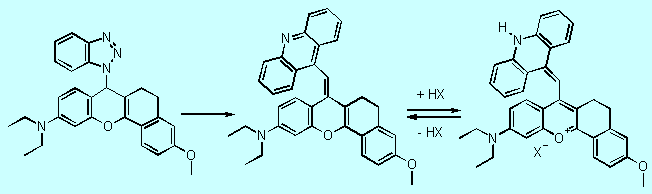
10 11 12
Although
a number of alternative synthetic routes to 11/12 have been explored,[
76KGS1484, 72JHC669]
only the benzotriazole-assisted methodology proved to be successful in
this case.[98uw-2]
Using benzotriazole methodology, we have developed the first general method to allow the indirect electrophilic substitution of various p-deficient O-, S-, and N-heterocyclic salts of fundamental importance. Introduction of various n-alkyl, benzyl or heteroaralkyl substituents in the position 4 to the heteroatom in 3 improved the solubility and stability and opens ways for further derivatizations. Depending on the substituent pattern of the starting materials 3, different products are obtained as colored, highly fluorescent and functional compounds of interest in high tech fields. These compounds may be investigated for use in the area of bischromophoric systems, Langmuir-Blodgett films or in analytical chemistry as pH-sensors or membrane probes, due to their amphiphilic nature.
Supporting Information Available: The varieties of products obtained
are shown on the general Schemes 2, 3, and 4. Experimental details and
NMR data of compounds 3, 5, 7-9 available as
a ISIS format database (ZIP file, 230
Kb). Both reaction and structure similarity searches are possible
for 91 compounds.
[69JOC1420] D. Redmore, J. Org. Chem. 1969, 34, 1420.
[72JHC669] J. A. Van Allan, G. A. Reynolds, J. Heterocyclic Chem. 1972, 9, 669
[72ZOB298] S. V. Krivun, O. F. Voziyanova, S. N. Baranov, Zh. Obshch. Khim. 1972, 42, 298 (Chem. Abstr. 1972, 77, 34224).
[72ZOB58] S. V. Krivun, O. F. Voziyanova, S. N. Baranov, Zh. Obshch. Khim. 1972, 42, 58 (Chem. Abstr. 1972, 77, 48587).
[74MI81] D. Basting, F. P. Schaefer, B. Steyer, Appl. Phys. 1974, 3, 81.
[75AGE581] R. R. Schmidt, Angew. Chem., Int. Ed. Engl. 1975, 14, 581.
[76KGS1484] E. A. Zvezdina, M. P. Zhdanova, V. A. Bren, G. N. Dorofeenko, Khim.Geterotsikl. Soedin. 1976, 1484.
[77DOK599] Yu. P. Andreichikov, N. V. Kholodova, G. N. Dorofeenko, Dokl. Akad. Nauk. SSSR (English Transl.) 1977, 236, 599.
[78BCJ2684] K. Ishikawa, K. Akiba, N. Inamoto, Bull. Chem. Soc. Jpn. 1978, 51, 2684.
[83BSF115] V. Wintgens, J. Kossanyi, M. Simalty, Bull. Soc. Chem. Fr. 1983, II, 115.
[83T3005] G. A. Iacobucci, J. G. Sweeny, Tetrahedron 1983, 39, 3005
[85JPC983] G. W. Fischer, J. Prakt. Chem. 1985, 327, 983.
[89BCJ2279] H. Kawara, S. Niizuma, Bull. Chem. Soc. Jpn. 1989, 62, 2279.
[89MI13] P. Czerney, G. Haucke, Applied Fluorescence Technology 1989, Vol. I, 13.
[89MI805] G. Haucke, P. Czerney, C. Igney, H. Hartmann, Ber. Bunsenges. Phys. Chem. 1989, 93, 805.
[90JA3845] M. R. Detty, P. B. Merkel, J. Amer. Chem. Soc. 1990, 112, 3845.
[90JST411] G. Haucke, P. Czerney, H. D. Ilge, D. Steen, H. Hartmann, J. Mol. Struct. 1990, 219, 411.
[91HOU30] P. Czerney, H. Hartmann, in Methoden Der Organischen Chemie (Houben Weyl), ed. by Kreher, R. P., Band E 7a; Heteroarene II, part 1, G. Thieme Verlag, Stuttgart - New York, 1991, pp. 30-170.
[91CB1809] A. R. Katritzky, X. Lan, J. N. Lam, Chem. Ber. 1991, 124, 1809.
[91T2683] A. R. Katritzky, S. Rachwal, J. Hitchings, Tetrahedron 1991, 27, 2683.
[92ZN1011] M. Bogatian, C. Deleanu, G. Mihai, T. S. Balaban, Z. Naturforsch.1992, 47B, 1011.
[92MI880] G. Haucke, P. Czerney, F. Cebulla, Ber. Bunsenges. Phys. Chem. 1992, 96, 880.
[93MI561] G. Haucke, P. Czerney, D. Steen, W. Rettig, H. Hartmann, Ber. Bunsenges. Phys. Chem. 1993, 97, 561.
[93Pat] P. Czerney, U. W. Grummt, H. Lindauer, G. J. Mohr, Ger. Offen. DE 4.341.618 (Cl. C09B23/01), Appl 07 Dec 1993 (Chem. Abstr. 1995, 123, 328758r).
[94CRV1063] M. A. Miranda, H. Garcia, Chem. Rev. 1994, 94, 1063.
[94DP229] H. Lindauer, P. Czerney, G. J. Mohr, U. W. Grummt, Dyes Pigm. 1994, 26, 229.
[94MI31] A. R. Katritzky, Z. Yang, D. T. Cundy, Aldrichimica Acta 1994, 47, 31.
[95JPC216] H. Lindauer, P. Czerney, U. W. Grummt, J. Prakt. Chem. 1995, 337, 216.
[95MI31] P. Czerney, G. Graness, E. Birckner, F. Vollmer, W. Rettig, J. Photochemistry and Photobiology A: Chemistry 1995, 89, 31.
[95MI247] M. Sauer, K. T. Han, R. Mueller, S. Nord, A. Schulz, S. Seeger, J. Wolfrum, J. Arden-Jacob, G. Deltau, N. J. Marx, C. Zander, K. H. Drexhage, Journal of Fluorescence 1995, 5, 247.
[96CHEC245] M. Balasubramanian and J. G. Keay in "Comprehensive Heterocyclic Chemistry II", vol. 5, ed. by A. R. Katritzky, C. W. Rees, E. F. V. Scriven, Pergamon, Oxford, 1996, pp. 245-300.
[96CHEC469] G. R. Green, J. M. Evans and A. K. Vong, "Comprehensive Heterocyclic Chemistry II", vol. 5, ed. by A. R. Katritzky, C. W. Rees, E. F. V. Scriven, Pergamon, Oxford, 1996, pp. 469-500.
[96MI] R. P. Haugland, "Handbook of Fluorescent Probes and Research Chemicals", 1996, Sixth Edition, Molecular probes Inc., Eugene. OR.
[96MI395] P. Czerney, U. W. Grummt, Sensors and Actuators 1996, B 38-39, 395.
[96RRC979] M. Bogatian, G. Mihai, M. Plaveti, F. Chiraleu, C. Deleanu, V. Badescu, T. S. Balaban, Rev. Roum. Chim. 1996, 41, 979.
[97JOC8198] A. R. Katritzky, P. Czerney, J. R. Levell, J. Org. Chem. 1997, 62, 8198.
[97H2413] A. R. Katritzky, S. Denisenko, P. Czerney, Heterocycles 1997, 2413.
[98CRV409] A. R. Katritzky, X. Lan, I. Z. Yang, O. V. Denisko, Chem. Rev. 1998, 409.
[98JOC3450] A. R. Katritzky, S. Denisenko, D. C. Oniciu, I. Giviriga, J. Org. Chem. 1998, 63, 3450.
[98JPCip] A. R. Katritzky, W. Du, S. N. Denisenko, P. Czerney, unpublished work.
[98uw] A. R. Katritzky, P. Czerney, J. R. Levell, W. Du, unpublished work.
[98uw-2] P. Czerney, V. Jesser, U.-W. Grummt, unpublished work.