| [Related articles/posters: 095 008 119 ] |
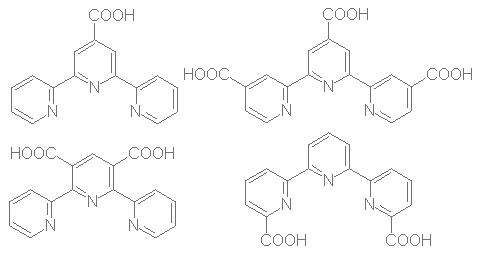 Scheme 1
Scheme 1
Direct introduction of a carboxylic group at a determined position of a terpyridine is a difficult task. We chose to introduce a furane as a potential carboxylic group, which allow us more handable materials and fair yields. The ligands are synthesized in four steps using Hantsch's method for pyridine ring formation. We show below (scheme 2) the general pathway used to build our ligands. Chalcone 1 is easily prepared by aldolisation between furfuraldehyde and the appropriate acetylpyridine according to litterature[2] with some yield improvement after optimisation. 1,4-Michaël addition of ethyl-3-(2-aryl)-3-oxopropanoic acid to 1 using diethylamine[3] gave 2 in moderate to fair yield and cyclization with ammonium acetate[4] allowed the formation of dihydropyridine 3. The latter is easily oxidized[5] to the corresponding pyridine 4. Strong oxidation of 4 with KMnO4 followed by acidification gave diacid 5 in moderate yield after recrystallization[6]. Yields and remarks are given in table 1.
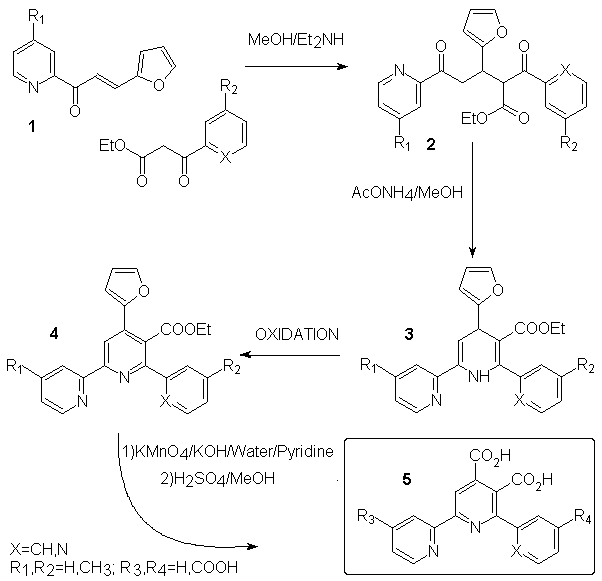 Scheme 2
Scheme 2
| Entry | Substituents | Yield (%) | Remarks |
| 1 | R1=H | quant. | m.p.=54°C (lit.=52-54°C) |
| 1' | R1=CH3 | 85 | light yellow cristals, m.p.=73-74°C |
| 2a | R2=H, X=N | 87 | |
| 2b | R2=CH3, X=N | 82 | |
| 2'b | R2=H, X=CH | 85 | |
| 3a | 96 | about 5% of 4a is formed together with3a | |
| 3b | not isolated | ||
| 3'b | 27 | ||
| 4a | 87 | start. from crude 3a, ox. with benzoquinone | |
| 4b | 85 | start. from crude 3b, ox. with benzoquinone | |
| 4'b | 35 | oxidation using MnO2 | |
| 5a | R3=R4=H | 35 | ES/MS and NMR in accordance |
In order to avoid coordination of the metal to the carboxylic acids during complexation and to make purification easier, we were brought to protect the acids as methyl esters using POCl3/PCl5 followed by methanolysis. Under these conditions, we have isolated a ring chlorinated compound in a fair amount (9%). We show below the products obtained when starting from compound 5a.
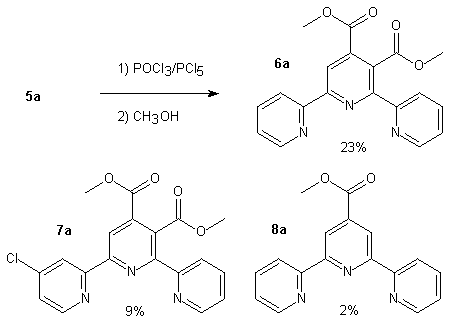 Scheme 3
Scheme 3
Conclusion
For the first time an unsymmetrical 2,2'-6'2"-terpyridine carrying two carboxylic acids (compound 5a) has been prepared in few steps and with a rather good overall yield compared to other terpyridines. We are currently investigating this reaction sequence with the other derivatives cited here. Some ruthenium complexes formed starting from 5a or 6a have yet been made and will be published later .
References
[1] Md.K.Nazeeruddin, P.Péchy and M.Grätzel, Chem.Commun.,1705,(1997);
F.H. Case and W.A. Butte, J.Am.Chem.Soc., 26, 4415, (1961);
J.P.Colin, University of Strasbourg, personnal communication.
[2] N.P.Buu-Hoï, N. Dat Xuong and T. Cong Trieu, Bull.Soc.Chim.Fr., 584, (1961).
[3] C.S.Marvel, L.E.Coleman and G.P. Scott, J.Org.Chem., 20, 1785, (1955);
R.Kuhn and H.R.Hensel, Ber., 86, 1333, (1953).
[4] F. Kröhnke, Synthesis, 1, (1976).
[5] Milton C. Kloetzel, Jack L. Pinkus and Robert M. Washburn, J.Am.Chem.Soc. 79, 4222, (1957).
[6] A.J. Fatiadi, Synthesis, 65 and 133, (1976), and references cited herein.