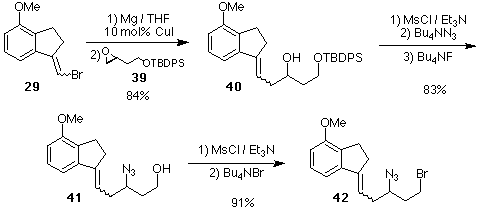
Scheme 9. Preparation of the Key Azido-Alkene 42.

Scheme 9. Preparation of the Key Azido-Alkene 42.
Treatment of 42 with triflic acid in benzene followed by sodium borohydride reduction of the resultant iminium ion generated a mixture of Schmidt products which were subjected, without purification, to bromide displacement using acetate ion. Again, without purification, the mixture of acetates was reduced with lithium aluminum hydride to produce a mixture of the desired alcohol 2, its stereoisomer 44, and the regioisomer 45 in good overall yield from 42. As in earlier attempts, the stereoselectivity of the reduction of the desired iminium ion was poor. We were able to overcome the nonselective reduction by switching to bulkier reducing agents. L-Selectride proved to be the most successful hydride reagent, ultimately producing the desired compound 2 as a single diastereomer (i.e., none of 44 was detected) accompanied by a small amount of the regioisomer 45. The overall yield of 2 from 42 using this procedure was 45%. Since the aromatic ring of 2 has been reduced by Ito, et. al[Ref.12] to afford an advanced intermediate in Kishi's synthesis of gephyrotoxin,[Ref.7] our synthesis of 2 constitutes a formal total synthesis of gephyrotoxin. The synthesis of 2 required 9 steps (5 purifications) from commercially available 4-methoxy-1-indanone 19, and proceeded in 22% overall yield.
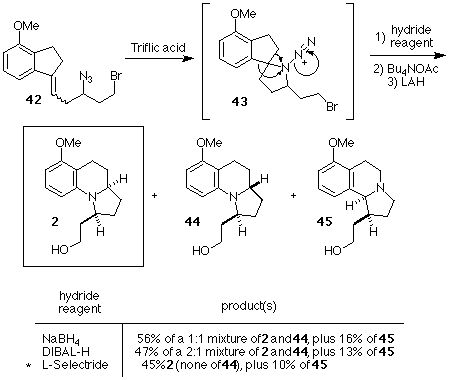
Scheme 10. Completion of the Formal Total Synthesis of Gephyrotoxin
1. Introduction
2. Model Studies: The Basic Schmidt Reaction,
wherein we study the regioselectivity of the rearrangement and the stereoselectivity
of the iminium ion reduction.
3. Initial Efforts to Synthesize Gephyrotoxin, wherein we struggle with the regioselectivity of the indene alkylation and the choice of a synthetic equivalent for the 2-hydroxyethyl side chain.
4. Formal Synthesis of Gephyrotoxin (THIS PAGE) wherein we finally get
it right.
5. References