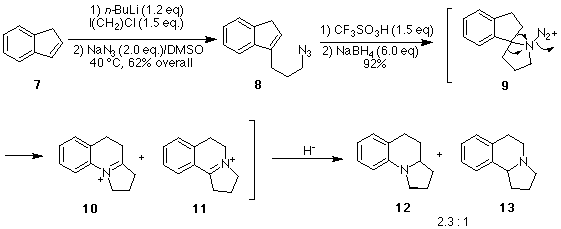
An important question to be resolved in our use of the intramolecular Schmidt
reaction was the issue of regioselectivity, i.e., which bond will migrate in the
intermediate aminodiazonium ion? Our ability to predict the regioselectivity of intramolecular
Schmidt reactions is still in its infancy. Thus, we set out to generate the simple
aminodiazonium ion 9 (Scheme 2) to explore whether
the aryl group, alkyl group, or perhaps even a hydrogen would migrate to the electron
deficient nitrogen. In an extension of this study, we explored the viability of a
secondary azide and the issue of the stereoselectivity of the iminium ion reduction
which arises (Scheme 3).
Scheme 2. Synthesis of the basic ring skeleton of gephyrotoxin.
B. The Use of a Secondary Azide: Stereoselectivity of
the Iminium Ion Reduction.
For the total synthesis of gephyrotoxin, a secondary rather than primary azide will
be required in the Schmidt precursor. Given the ease of assembly of the cyclization
precursors using the indene alkylation method, a simple model study was conducted
to ascertain whether a secondary azide can be used in intramolecular Schmidt reaction
(Scheme 3). This study also addresses the question
of the stereoselectivity of the reduction of the resultant iminium ion.
Metalation of indene (7) and alkylation with the chloride 14 [Ref.20]
gave the alcohol 15 in good yield. Mesylation followed by azide displacement
provided the desired azido-indene 16 in 58% yield. Treatment of 16
with triflic acid in benzene afforded a mixture of iminium ions that were reduced
with sodium borohydride to give the desired tricyclic aniline 17 (resulting
from phenyl migration) and the benzylic amine regioisomer 18 (resulting from
alkyl migration in 72% combined yield. The iminium ion reduction had proceeded with
poor stereoselectivity, producing 17 as a 1:1 mixture of diastereomers. However,
we hoped that a bulkier hydride reagent in combination with a bulky alcohol protecting
group on the 2-hydroxyethyl side chain of the "real" system would lead
to an improvement in the stereoselectivity of the iminium ion reduction.
Scheme 3. Use of a Secondary Azide in the Schmidt Reaction. Stereoselectivity of Iminium Ion Reduction.
1. Introduction
2. Model Studies: The Basic Schmidt Reaction, (THIS PAGE), wherein we
study the regioselectivity of the rearrangement and the stereoselectivity of the
iminium ion reduction.
3. Initial Efforts to Synthesize Gephyrotoxin, wherein we struggle with the regioselectivity of the indene alkylation and the choice of a synthetic equivalent for the 2-hydroxyethyl side chain.
4. Formal Synthesis of Gephyrotoxin, wherein
we finally get it right.
5. References