| [Molecules: 12] [Related articles/posters: 001 018 024 039 052 ] |
![]()
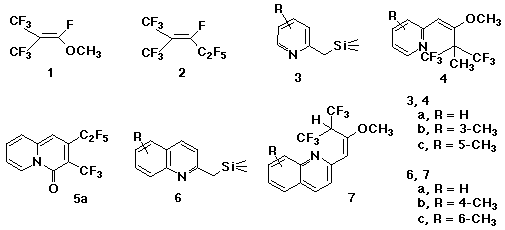
In order to compare the reactivity of methyl perfluoro(2-methylprop-1-enyl) ether 1 with perfluoro(2-methylpent-2-ene) 2, the reaction of 1 with 2-(trimethylsilylmethyl)pyridines 3 and their analogues was performed. When a mixture of 2-(trimethylsilylmethyl)pyridine 3a and potassium fluoride was treated with 1 in THF, a methylated adduct, 2-[2-methoxy-3,3-bis (trifluoromethyl)but-1-enyl]pyridine 4a, was obtained in 50% yield instead of a cyclized adduct, 2-pentafluoroethyl-3-trifluoromethyl-4H-quinolizin-4-one 5a, which was given from 2.1 However, 2-(trimethylsilylmethyl)quinoline 6a gave an adduct, 2-(4,4,4-trifluoro-2-methoxy-3-trifluoromethylbut-1-enyl) quinoline 7a in 58% yield, accompanied by 23% of the corresponding methylated adduct. The analogous reaction of 2-(trimethylsilylmethyl)benzothiazole is also discussed.