![]()
![]()
![]()
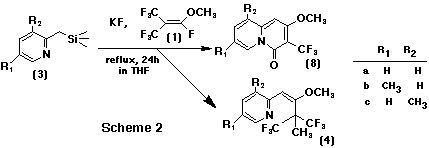
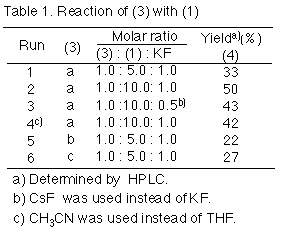
On treating a mixture of 2-(trimethylsilylmethyl)pyridine 3a and 1 mol equiv. of potassium fluoride with 5 mol equiv. of 1 in tetrahydrofuran (THF) at -5oC for 1 h, and then stirring it for 24 h under reflux, a methylated adduct, 2-[2-methoxy-3,3-bis (trifluoromethyl)but-1-enyl]pyridine 4a, was obtained in 33% yield instead of a cyclized compound, 2-methoxy-3-trifluoromethyl-4H-quinolizin-4-one 8. This was an analogue of 2-pentafluoroethyl-3-trifluoromethyl-4H-quinolizin-4-one 5a, which was given from perfluoro(2-methylpent-2-ene) 2.1 Excess of 1 (10 mol equiv.) enhanced the yield of 4a to 50%. Addition of caesium fluoride, instead of potassium fluoride, gave an analogous result (43% yield), and acetonitrile is also suitable as a solvent for this reaction. Furthermore, the reaction was applied to the 5-methyl- and 3-methyl derivatives, 3b and 3c, to give the corresponding adducts 4b,c in 22 and 27 % yields, respectively.