| [Molecules: 10] [Related articles/posters: 007 018 024 063 112 ] |
Faculty of Engineering, Shinshu University, Wakasato 500, Nagano 380, Japan
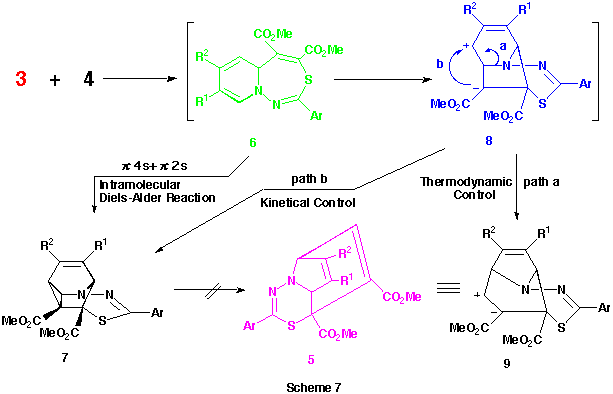
Recently, we reported a first syntheses of dimethyl 10aH-pyrido[1,2-d][1,4]thiazepine-1,2-dicarboxylate derivatives from the reactions of various 1-pyridinio[(substituted thiocarbonyl)methylides] with dimethyl acetylenedicarboxylate (DMAD) and their smooth transformations to the intramolecular Diels-Alder adducts, dimethyl 4-thia-1-azatetracyclo[5.4.0.05,11.06,8]undeca-2,9-diene-5,6-dicarboxylates. More recently, we also proved that the reactions of the 1-pyridiniomethylides with DMAD proceed stepwise via the formation of primary 1 : 1 zwitterionic intermediates such as A (see Scheme 1) followed by their 1,7-cyclization, since the trapping of the intermediates using various alcohols, which were added into the reaction mixtures, and the intramolecular Diels-Alder reactions of the resulting adducts gave quite different types of products, 2-alkoxy-6-thia-3-azatricyclo[5.3.1.03,8]undeca-4,9-diene derivatives.
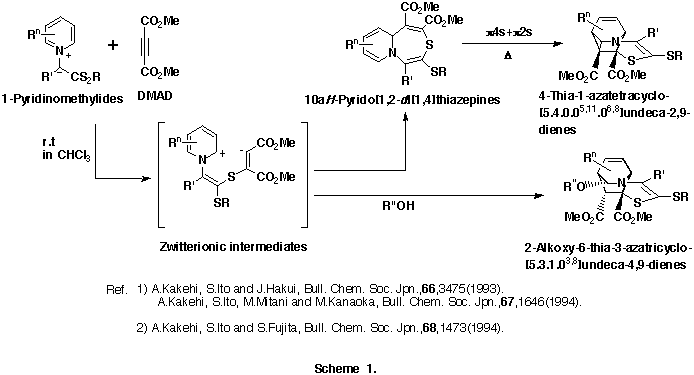
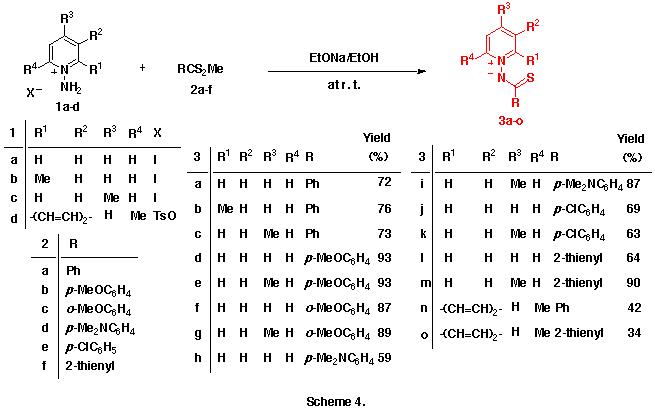
As an extension of this reaction, we were interested in the reactions of 1-pyridinio(substituted thiocarbonyl)aminides with DMAD. Our previous efforts to isolate any significant products from the reactions of 1-pyridinio[(methylthio)thiocarbonyl]aminides with DMAD under various reaction conditions were fruitless. The initially expected 5aH-pyrido[1,2-d][1,3,4]thiadiazepines and/or 4-thia-1,2-diazatetracyclo[5.4.0.05,11.06,8]undeca-2,9-dienes could not be obtained, and trace amounts of dimethyl fumarate and hexamethyl benzenehexacarboxylate only were detected. (Scheme 2)
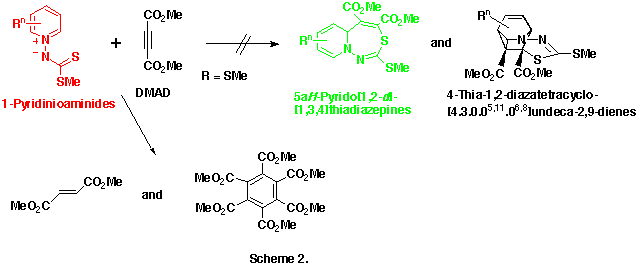
We explained these unsuccessful results as follows
In order to confirm the second assumption we modified the structure of 1-pyridinioaminides, and their reactivities toward DMAD were examined.
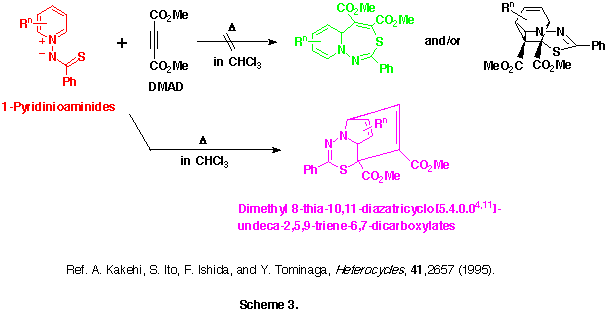
Interestingly, the reactions of 1-pyridinio(thiobenzoyl)aminides with DMAD in dry and alcohol-free chloroform at 50-60 oC gave neither the initially predicted 5aH-pyrido[1,2-d][1,3,4]thiadiazepines nor their intramolecular Diels-Alder adducts, but they afforded quite different types of products, dimethyl 9-phenyl-8-thia-10,11-diazatricyclo[5.4.0.04,11]undeca-2,5,9-triene-6,7-dicarboxlates, in moderate yields. (See Scheme 3)
Here, we will describe the application of this reaction to various 1-pyridinio-(arylthiocarbonyl)aminides and on first isolation of two title compounds, 5aH-pyrido[1,2-d] [1,3,4]thia-diazepine derivatives. Their possible reaction mechanisms involving pyridothiadiazepine and 4-thia-1,2-diazatricyclo[5.4.0.05,11]undeca-2,9-dien-8-yl-6-ide intermediates will be also discussed.
As observed in the reactions of 1-pyridinio[(substituted thiocarbonyl)methylides] with DMAD, these reactions seems to proceed via the formation of 5aH-pyrido[1,2-d][1,3,4]thiadiazepine derivatives such as 6 and their rearrangements. Here, we presumed the presence of the 4,9-cyclized intermediate, 4-thia-1,2-diazatricyclo[5.4.0.05,11]undeca-2,9-dien-8-yl-6-ide (see below) from these 5aH-pyrido[1,2-d][1,3,4]-thiadiazepine derivatives 6 in the latter rearrangement, and planned the syntheses and the isolation of benzo-fused 5aH-pyrido[1,2-d][1,3,4]thiadiazepine derivatives in which this transition state is energitically less favourable. As might be expected, the reactions of 1-(2-methylquinolino)(phenyl- 3n and 1-(2-methylquinolino)(2-thienylthiocarbonyl)aminide 3o with 4 gave smoothly the corresponding dimethyl 5a-methyl-2-phenyl- 6n and 2-(2-thienyl)-5aH-1,3,4-thiadiazepino[4,5-a]quinoline-4,5-dicarboxlate 6o in 41 and 43% yields, respectively , as yellow crystals. (Scheme 6) Compounds 6n,o were very unstable and decomposed gradually at room temperature.
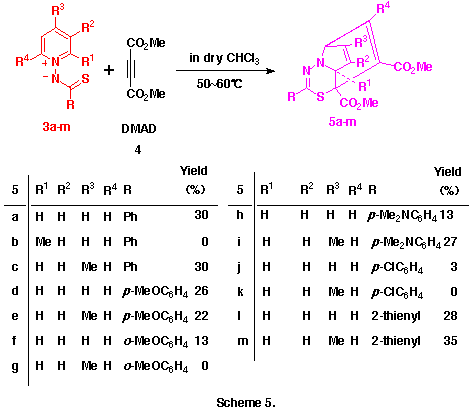
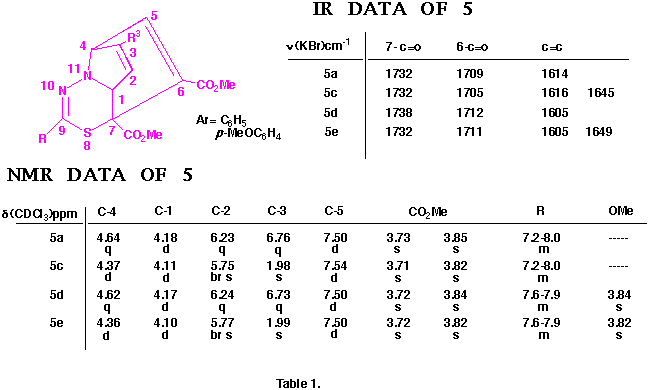
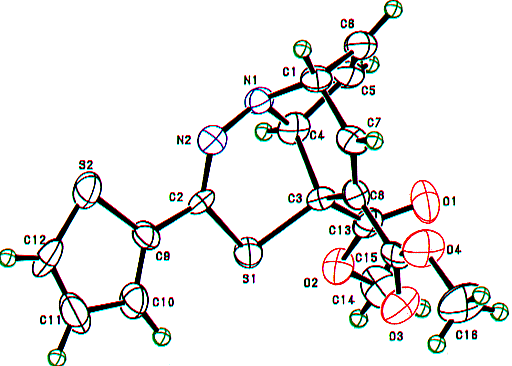
Figure 1
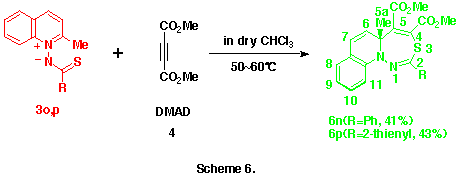
Similarly, the structures of the above thiadiazepinoquinolines 6n,o were assigned by theri elemental analyses, and IR and NMR spectroscopy (see below), and finally comfirmed by the X-ray analysis of 6n. The ORTEP drawing for 6n is shown in Figure 2.
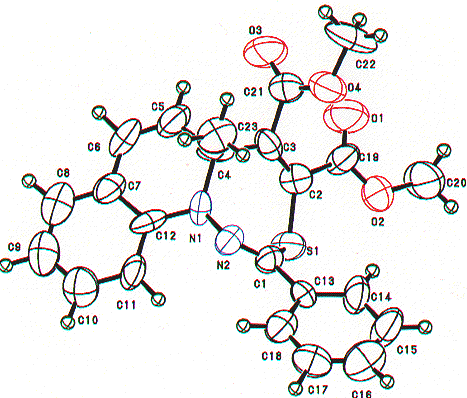
Figure 2
The reason why only this type of compound 5a-m was obtained in the reactions of aminides 3a-m with 4 while other types of product such as 6 and 7 were not is still unclear. However, the facts that these reactions did not give any significant products at room temperature but, only on heating, the corresponding compounds 5 could be obtained may indicate the following.