 ECHET96 Article 055: John Lyga
ECHET96 Article 055: John Lyga
 message to the question about article 55 from John Lyga Hideshi Nakamua
message to the question about article 55 from John Lyga Hideshi Nakamua
Synthesis of 3,5-disubstituted 2-aminopyrazines by palladium-mediated
cross-couplings and its use for preparing chemi- and/or bio-luminescent
compounds
Hideshi Nakamura, Daisuke Takeuchi, Mihoko Aizawa, Chun Wu and Akio
Murai
Division of Chemistry, Graduate School of Science, Hokkaido University,
Sapporo 060, Japan
Introduction
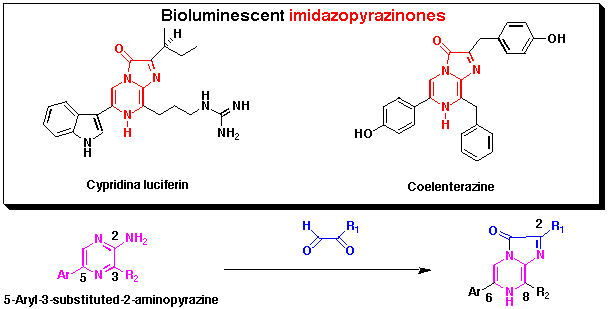
3,6,8-Trisubstituted imidazopyrazine-2-ones are widely distributed in marine organisms
and in some cases are used as bioluminescent substances such as a jellyfish Aequorea aequorea
and a crustacean Cypridina (Vargula) hirgendorfii. These compounds are also chemiluminescent
and utilized to detect chemicals such as Ca2+ or active
oxygen species such as superoxide. We have investigated convergent synthesis
in order to prepare imidazopyrazinones with different luminescent properties such as colour of luminescence.
Since 5-aryl-3-substituted-2-aminopyrazines have been used to prepare chemi- and/or
bio-luminescent imidazopyrazinones, we have developed a new approach to synthesize
3,5-disubstituted 2-aminopyrazines having conjugated chromophores from commercially
available 2-aminopyrazine by several steps including palladium-mediated cross-coupling as a key reaction.
Results and discussion
I Synthesis of 3,5-disubstituted 2-aminopyrazines from 2-aminopyrazine.
- Alkylation of 2-aminopyrazine with organolithium
- Arylation of 3-bromo and 3,5-dibromo-2-aminopyrazine
- Alkynylation of 3-bromo and 3,5-dibromo-2-aminopyrazine
II Synthesis of chemi- and/or bioluminescent imidazopyrazinones
- Synthesis of Coelenterazine
- Synthesis of Cypridina luciferin analogue
- Synthesis and chmemiluminescence of imidazopyrazinones
Conclusion
2-Aminopyrazine is shown to be a useful starting material for preparing various types of chemi- and bio-luminescent imidazopyrazinones which allow one to control luminescet properties such as colour and the rate determining steps. The method described above may lead to bioluminescent analogues by a short synthesis, which could be used to investigate the detailed mechanisms of bioluminescence. Bioluminescent compounds together with bioluminescent enzymes will provide a new tool for studies on time-dependent biological phenomena.
