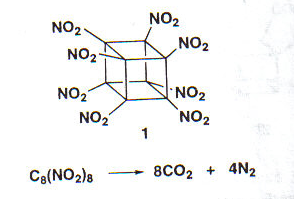
Cubane has the
highest strain energy (166kcal/mol) of any organic compounds available
in multi gram amount. It is a kinetically stable compound and only decomposite
above 220 Celsius Degree. It is also one of the most dense hydrocarbons
ever know.
However, although many physical properties of cubane have been measured,
in 1980 and before, cubane was considered just a laboratory curiosity
of interest only to academics. It changed, in early 1980s when Gilbert
of U.S Army Armament and Development Command (now ARDEC) pointed out that
cubane's very high heat of formation and its exceptionally high density
could make certain cubane derivatives important explosives.
|