

|
||||||||||||||||||||||||||||||
| |
||||||||||||||||||||||||||||||
Application of cubane derivatives: |
||||||||||||||||||||||||||||||
Pharmaceutical aspect of cubane |
||||||||||||||||||||||||||||||
| Because the cubane frame is rigid, substituent have precise spatial relationships to each another. The distance across the cubane (the body diagonal) is almost the same as that between the para positions of the benzene ring. On cubane, on can add substituents in the "benzene plane", as well as above and below it, so to speak. This offers fascinating position possibilities for the synthesis of new pharmaceuticals. A number of cubane derivatives have already been obtained which shows interesting activity in anti-AIDS and anti-tumor screens. Although the activity or the toxicity balance of cubane is yet not know, the cubane system is not inherently toxic. Most of cubanes are biologically innocuous. The research of cubane pharmaceutical has just began. At least now, cubane is a biologically stable, lipophilic platform on which the chemist can install a wide range of substituents in a variety of well defined special relationships. Developments in drug design programs should allow the judicious choice. | ||||||||||||||||||||||||||||||
 |
Dipivaloylcubane: a cubane derivatized with keto, cyano, and amide groups, shown on the left- exhibits moderate activity against human immunodeficiency virus (HIV), which causes AIDS, without impairing healthy cells. | |||||||||||||||||||||||||||||
Polymers of cubane: |
||||||||||||||||||||||||||||||
Optically transparent cubanes and cubylcubanes have been proposed as building blocks for rigid liquid-crystal compounds. UV active cubanes, for example cubyl ketones, are readily transformed photochemically into coloured cyclooctatetraenes;this transformation can be used to permanent information storage. |
||||||||||||||||||||||||||||||
 |
Another example of UV active cubane, which can be used to synthesis liquid crystals. | |||||||||||||||||||||||||||||
| Polymers with cubane in the backbone or as a pendant group along a polymer chain is focused now. The cubane subunits in these polymers can be rearranged easily to cycloctatetraenes. It is expected that polycyclooctatetra can be converted in to polyacetylenes by the way of ring-opening metathesis polymerization. The polyacetylenes will have properties which are enhanced by the chain being intrinsically part of another polymer. These properties including stability and extrudability and etc. A example is shown below: | ||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
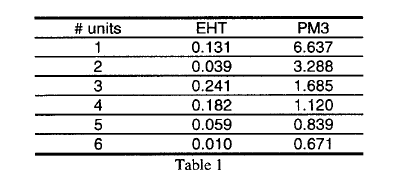
Cubane derivative could be the structural basis for a class of intrinsic small gap polymers. The small gap polymer could present intrinsic good conductivity without doping, good nonlinear optical and photoelectric properties. Investigation of oligamers with up to six units of a conjugated unsaturated cubane derivative, where all the hydrogen were removed, is carried out. The table below shows that the gap values in eV by EHT and PM3. These values suggest to us that these structures could be used to design a new class of polymers with very small gap. |
||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
Explosive and fuels: |
||||||||||||||||||||||||||||||
| In the early 1980s Everett Gilbert of the U.S. Army Armament Research and Development Command (now ARDEC) pointed out that the nitrocarbon octanitrocubane (ONC), then unknown, has a perfect oxygen balance, and in light of the properties of the parent hydrocarbon cubane should have a very high heat of formation per CNO2 unit and an exceptionally high density as well. His colleagues Jack Alster, Oscar Sandus and Norman Slagg at ARDEC provided theoretical support for Gilbert's brilliant insight and estimated that ONC would have a detonation pressure significantly greater than HMX. Later, both statistical and computational approaches predicted a density of 2.1 ± 2.2 g /cm3 for octanitrocubane, greater than any other C, N, O compound. | ||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
| For a more detail look at explosive side of cubane derivatives, please refer to the discussion section of this website. | ||||||||||||||||||||||||||||||