Similar Drugs:
Other similar drugs include the beta blockers:
Salmeterol: 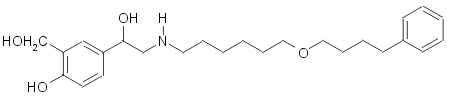
Formoterol: 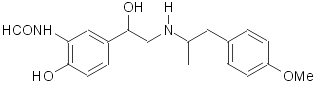
And the less receptor specific:
Isoproterenol: 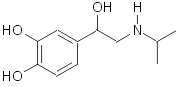
Epinephrine (adrenaline): 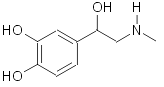
Norepinephrine (noradrenaline): 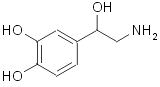
Other asthma treatments:
Other than beta bloackers another common asthma treatment is the used
of inhaled (or in some cases injected) corticosteroids. Corticosteroids
are the most potent and effective long term medication for asthma.
The effect of corticosteroids include reduced severity of symptoms, improved
peak expiratory flow, reduced airway hyperresponsiveness and prevention
of asthma attacks. Corticosteroids produce a number of pharmacological
effects important in the inhibition of the inflammatory processes,
though the precise mechanisms by which these effects are produced remain
elusive.
Future drug development:
The furture of asthma drugs appears to be Leukotriene synthesis inhibitors.
T-lymphocytes produce interleukin-4, promoting maturation of B-lymphocytes
into plasma cells in the first step of the inflammation caused by asthma.
There are four types of gents targeting leukotriene activity:
- Leukotriene synthesis inhibitors - zileuton
- cysteinyl leukotriene receptor antagonists - zafirlukast, pranlukast and montelukast
Leukotrienes, discovered in 1979, ultimately mediate the production of IgE, and nonspecific bronchial hyperresponsiveness may also be due to the priming of airway smooth muscle by leukotrienes. The cysteinyl leukotrienes induce bronchoconstriction and stimulate both mucous secretion and microvascular leakage to contribute to airway obstruction. There have been numerous complications in bringining agents targeting leukotriene activity to the market however there are definately the single biggest marketed innovation in asthma therapy in many years. Instead of merely treating symptoms with bronchodilators or broadly suppressing the immune response with corticosteroids, these agents allow interruption of asthma's pathogenesis much farther up its chain of events with much tighter focus and thus less side effects.
The first product to under go clinical trials was Venzair (by Merck)
in 1991, but was rejected because of its tendency to cause liver damage.
Leutrol (A zileuton) suffered a similar fate in 1995, but has since been
approved (as Zyflo) with cautions to monitor liver function. A zafirlukast
marketed by Zeneca (Accolate) started selling in November 1996 and a longer
acting variation (Singulair a montelukast from Merck) was approved
in late 1997. A pranlukast from SmithKline Beecham's (Ultair) is already
on the Japanese market, while tomelukast, pobilukast and verlukast are
currently being researched (if not already launch by the time this
is read).