| [Related articles/posters: 023 016 011 ] |

| (a) Inorganic Chemistry 1, Chemical Center, Lund University, P.O. Box 124, S-22100 Lund, Sweden
(b) Dipartimento di Chimica "G.Ciamician", Università di Bologna, via Selmi 2, I-40124 Bologna, Italy | 
|
Transition metal carbonyl clusters can be used as catalysts themselves
or as models for metal surfaces in the process of chemisorption
in catalytic processes.1 One advantage
of using transition metal carbonyl clusters to model surface reactions
is that it is possible to study metal-ligand interactions in detail
through analytical techniques not available to surface scientists,
e.g. NMR and IR spectroscopy, mass spectrometry, and X-ray
and neutron crystallography.
As an approach to the modelling of hydrodesulfurization (HDS)
reactions, we have investigated the reactions and coordination
modes of thiols to trinuclear osmium clusters. Dodecacarbonyl-triosmium
clusters are known to react with thiols to produce the trinuclear
compounds [HOs3(CO)10(SR)].2
We have studied the reaction between different thiols and the
acetonitrile substituted osmium cluster [Os3(CO)11(CH3CN)].3
The acetonitrile ligand is more labile than the carbonyls and
it is therefore easily substituted by suitable nucleophiles at
room temperature. Furthermore, the acetonitrile ligand may be
used as a kinetic probe for mass retardation studies and for proton
NMR investigations.
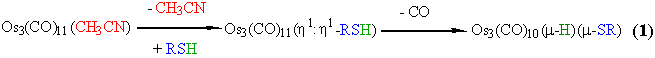
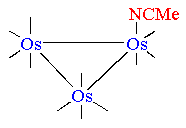
We have carried out the above reaction with a variety of different
thiols. All the resulting complexes are
very similar, their metal-carbonyl cores being basically isostructural.
Below is reported the molecular structure of the para-thiocresol
adduct.
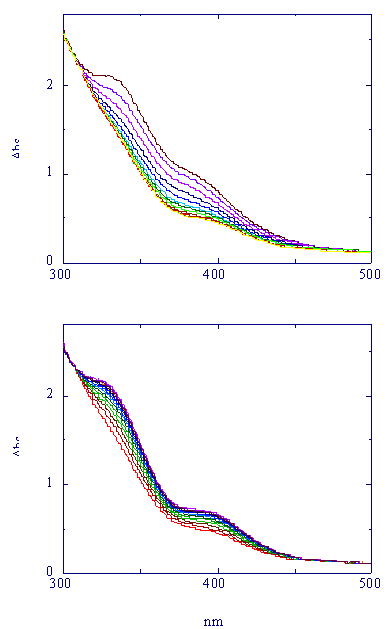
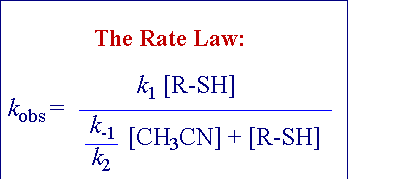
Systematic kinetic studies for these reactions were performed
with different ligand concentrations in pseudo-first order conditions,
and were followed spectrophotometrically. In the following picture
is reported the UV-visible spectral variation for the two-steps
reaction (1) between the triosmium cluster
and phenylthiol.
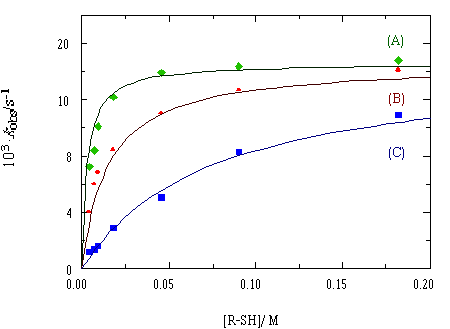
The plot of the rate constant for the para-thiocresol addition
at different acetonitrile concentrations shows a saturation
shape, with a curvilinear dependence on the concentration
of the entering thiol.
We have also carried out kinetic studies with
the para-thiocresol ligand by 1H-NMR spectroscopy
in the presence of different amounts of free acetonitrile, which
made it possible to follow both processes. The plot of the time
dependence of the relative concentration (I %) of the species
involved in the consecutive reactions is reported
below.
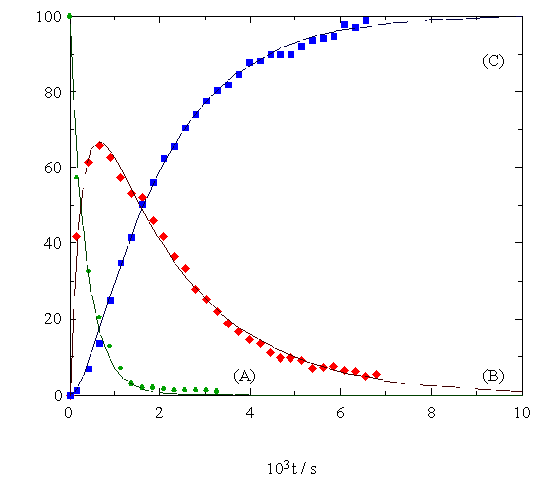
 ). B: [Os3(CO)11(h1:h1-SRH)]
(
). B: [Os3(CO)11(h1:h1-SRH)]
( ). C: [Os3(CO)10(m-SR)(m-H)]
(
). C: [Os3(CO)10(m-SR)(m-H)]
( ) .
) .
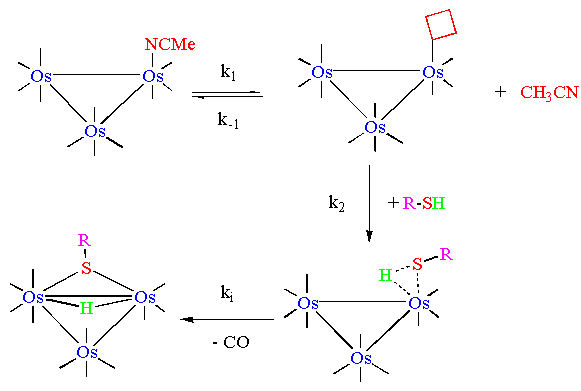
The observed kinetics indicate that both steps in the proposed
reaction mechanism are dissociative:
NMR relaxation experiments indicate that the mechanism of thiol addition proceeds via an intermediate in which an agostic Os-S-H interaction is detected. In the case of the para-thiocresol, the relaxation time T1 is 2.1 ± 0.2 ms for the intermediate and 2.7 ± 0.2 ms for the final species. The position of the h1-H signal is at lower field than that expected for a terminal hydride (d = - 4.65 ppm), especially if compared to the bridged resonance m-H (d = -17.0 ppm).
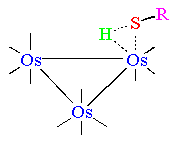
This is the first example of such an intermediate and may be related to the initial steps of HDS reactions on metal surfaces.