| [Related articles/posters: 028 011 019 ] |
|
|
[Related |
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565, Japanü@
After various attempts for achieving asymmetric intramolecular C-H/olefin coupling reactions, the monodentate ligand (R)-(S)-PPFOMe was found to be suitable for the rhodium-catalyzed asymmetric cyclization of 1-(2-pyridyl)-1E,5Z-diene (1). The diene 1 underwent cyclization in the presence of di-m-chloro-bis[bis(cyclooctene)rhodium(I)] (5 mol%) and (R)-(S)-PPFOMe (30 mol%) in THF (0.2 M) at 120 üŗC (oil bath temperature) for 1 h under nitrogen to give 2 in 78% yield and 28% ee (eq 1).
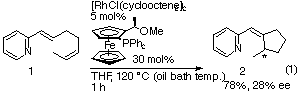
Imidazole derivatives are also applicable to the present reaction. When imidazolyl diene 3 reacted, cyclized product 4 was obtained in significantly higher optical yield. In this case, the enantioselectivity was further improved by lowering the reaction temperature. The unusual mild reaction conditions for this C-H/olefin coupling reaction, i.e., at room temperature, are especially noteworthy (eq 2).

We will describe our recent progress in this area of rhodium-catalyzed intramolecular cyclizations.