| [Related articles/posters: 005 008 020 ] |


 Bill Brower
Bill Brower Doug Armstrong
Doug Armstrong
- Whether it be for commercial collection or ecological purification, the competent ionophoric extraction of small metal cations is a phenomenon worthy of investigation. Dependent upon which ion is targeted, ring radius and heteroatomic configuration are variable factors which can be manipulated to achieve optimum customization. The smaller crown ethers are not useless. However, it is the 14-crown-4 that has received the bulk of commendatory research. Four such structures which have displayed extractive proficiency will be investigated. They are found in the research of Richard Bartsch, Hiroshi Tsukube, and Shijiro Ogawa. The optimal ionophore may yet be engineered, for none have shown unrivalled efficiency.
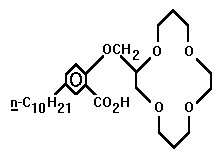
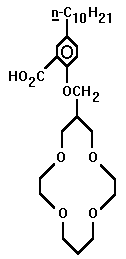
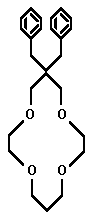
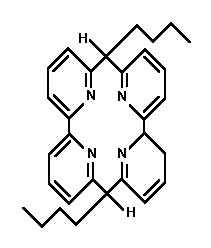
The complete overall structure of the ring is important when theorizing the flexibility of the ionophore. The nitrogen heteroatoms may be more selective than an oxygen-dominated crown ether. The Tsukubian amine side arm may prove more useful in regards to this aza-attractiveness, as well. When engineered ideally, after all these factors are fine-tuned, the smaller rings, such as the 9C3, 12C3, or 12C4, may prove more viable. The numerous factors and variables allow the search for the ideal ring to continue.