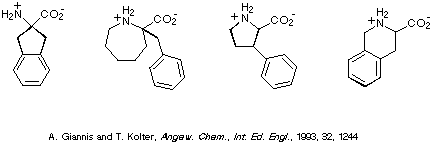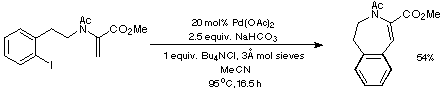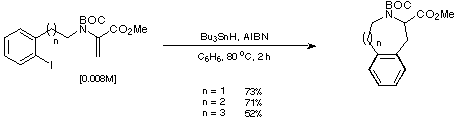Transition Metal Mediated Synthesis of Conformationally Constrained Phenylalanine Analogues
and Their Applications
Susan E. Gibson (née Thomas),a Nathalie
Guillo,a Richard J. Middletona and Matthew J.
Tozerb
Department of Chemistry, Imperial College of Science,
Technology and Medicine, South Kensington, London SW7 2AY, UK
b James Black Foundation, 68 Half Moon
Lane, Dulwich, London SE24 9JE
This contribution describes our recent work on the synthesis of
a new series of conformationally constrained analogues of phenylalanine
together with the first example of an application of the series in a bioactive
system. After an introductory outline of some of the biological aspects of
conformationally constrained amino acids, the synthesis of the conformationally
constrained phenylalanines using routes based on i) palladium catalysed Heck
cyclisations, and ii) radical cyclisations will be described. This will be
followed by a description of an application of this series of compounds which
will detail i) the chemical synthesis of several potential
cholecystokinin-B/gastrin receptor antagonists, and ii) the results of the
biological tests carried out on the compounds produced.
INTRODUCTION
Restricting the conformational freedom of peptides may lead to i) increased
binding affinity at receptors, increased selectivity at receptors, iii)
increased stability with respect to enzymatic degradation, and iv) increased
insight into the bioactive conformation of the peptide. One method of
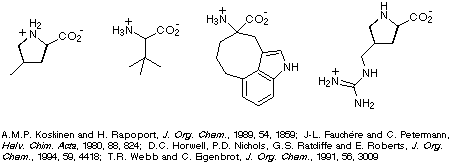
constraining the conformational freedom of peptides (or peptide derivatives) is
to introduce conformationally constrained amino acids, four examples of which
are illustrated in Scheme 1.
Scheme 1
Focussing on phenylalanine, several examples of conformationally
constrained analogues of this amino acid are depicted in Scheme 2. Of
particular note, in the context of this contribution, is the compound on the
far right i.e. 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid,
commonly referred to as Tic.
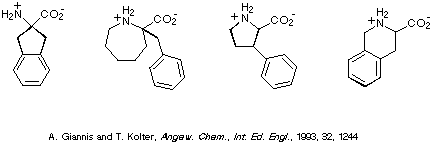
Scheme 2
Incorporation of Tic into a peptide not only restricts the conformational
freedom of the aromatic ring but also places constraints on the peptide
backbone. These effects provide valuable insight into the bioactive
conformation of the peptide ligand and often lead to potent and selective
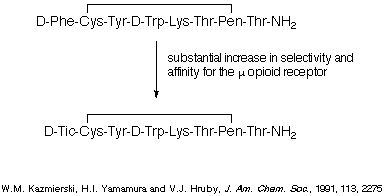
compounds. For example, replacement of a Phe residue with a Tic residue in the
octapeptide depicted in Scheme 3 led to a substantial increase in affinity and
selectivity for the u opioid receptor, and the dipeptide represented in Scheme
4 is currently showing significant promise in in vivo tests.
Scheme 3

Scheme 4
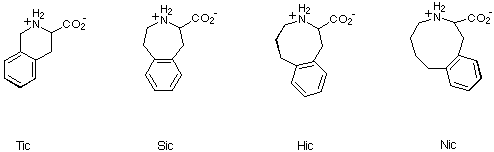
In view of the extensive use of Tic in studies of this type, we
envisaged that 2,3,4,5-tetrahydro-1H-3-benzazepine-2-carboxylic acid
(Sic), 1,2,3,4,5,6-hexahydro-3-benzazocine-2-carboxylic acid (Hic) and
2,3,4,5,6,7-hexahydro-1H-3-benzazonine-2-carboxylic acid (Nic), together
with Tic, would provide a useful series of compounds for probing bioactive
conformations of peptide or peptide-derived ligands (Scheme 5). We thus
embarked on the synthesis of Sic, Hic and Nic in order to test our
hypothesis.
Scheme 5
SYNTHESIS OF SIC, HIC AND NIC
Our retrosynthetic analysis of the cyclic amino acids Sic, Hic and Nic is shown
in Scheme 6.
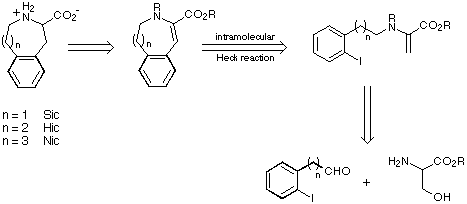
Scheme 6
Our initial targets were thus a series of iodoaldehydes, the synthesis
of which was carried out using standard procedures as depicted in Scheme 7.
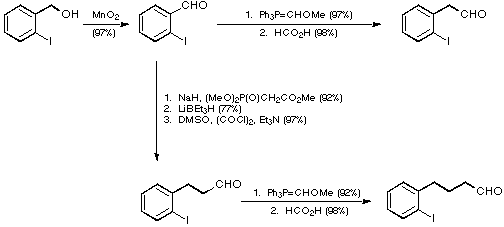
Scheme 7
Reductive amination of the iodoaldehydes with (+/-)-serine methyl ester was
then followed by either Ac or BOC protection and the introduction of a double
bond to give two sets of substrates for the proposed intramolecular Heck
reaction (Scheme 8).
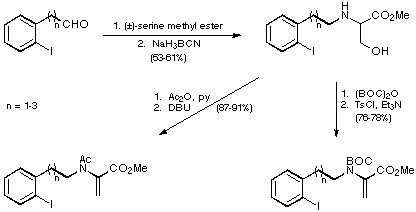
Scheme 8
At this point, it seemed appropriate to consider the cyclisation mode
that the proposed Heck reactions would follow i.e. would an endo
pathway dominate to give intermediates with b-hydrogens that would allow
the Heck cyclisation to proceed to give the required products, or would an
exo pathway be favoured leading to intermediates without appropriate
b-hydrogens which would possibly not only give undesired products but also
consume precious palladium catalyst (Scheme 9)?
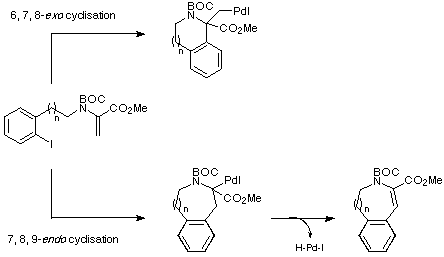
Scheme 9
A literature survey at this point in the project revealed that
although there were several hundreds of reported examples of intramolecular
Heck reactions giving five- and six-membered rings (almost always via an
exo mode of cyclisation), and some examples of the formation of
macrocycles, examples of the formation of medium-sized rings were extremely
rare (Scheme 10). Furthermore, from the small number of examples known it was
unclear which mode of cyclisation was favoured.
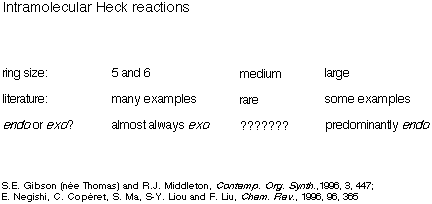
Scheme 10
The outcome of one of our many early Heck reactions is depicted in Scheme 11.
Relatively harsh conditions and use of Et3N as a base resulted in a
mixture of products derived from both endo and exo modes of
cyclisation.
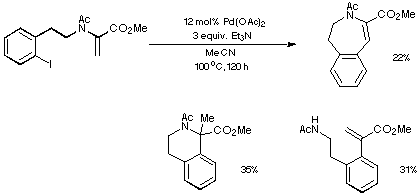
Scheme 11
In contrast, the somewhat milder conditions defined in Scheme 12 using
NaHCO3 as the base led to only the isolation of the desired
endo product.
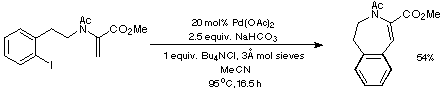
Scheme 12
An explanation for these observations is depicted in Scheme 13. It is
envisaged that the Et3N acts as a souce of hydride (an established
process) and so opens up the pathways leading to the exo products. In
the absence of Et3N these pathways cannot be accessed and only the
endo product is observed. However, the exo cyclisation still
occurs leading to a build-up of the palladium intermediate lacking
b-hydrogens (or a derivative thereof) and the consumption of palladium
catalyst.
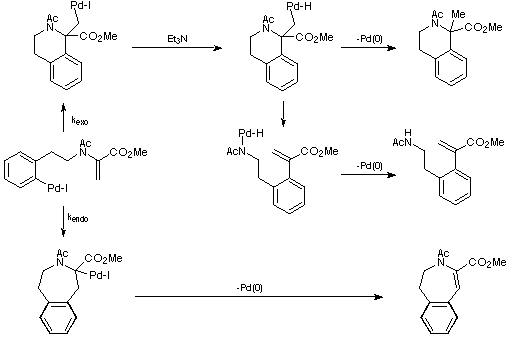
Scheme 13
This interpretation suggests that as the ring size increases and the
endo mode of cyclisation becomes more favourable than the exo
mode of cyclisation (for stereoelectronic reasons), then the reaction should
require less catalyst and give higher yields. This prediction is consistent
with the results depicted in Scheme 14 which illustrate that decreasing amounts
of palladium catalyst are required to generate essentially the same yield of
product as the ring size increases.
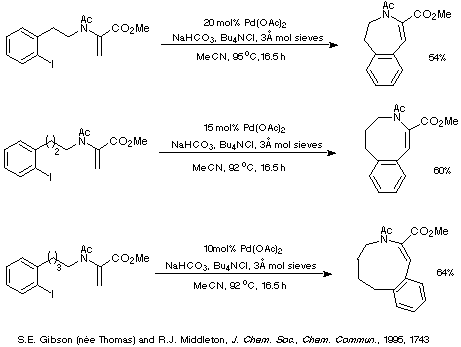
Scheme 14
At this stage in the project, although we were pleased that the
intramolecular Heck reaction was providing the seven-, eight- and nine-membered
rings we desired, we were still concerned about the quantity of palladium
catalyst that was being consumed. We thus carried out an extensive set of
experiments designed to optimise the conditions with a view to reducing the
catalyst loadings. The results of our experiments with the seven- eight- and
nine-membered rings are depicted in Schemes 15-17 respectively and summarised
in Scheme 18.
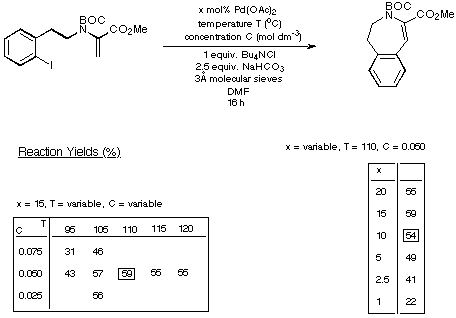
Scheme 15
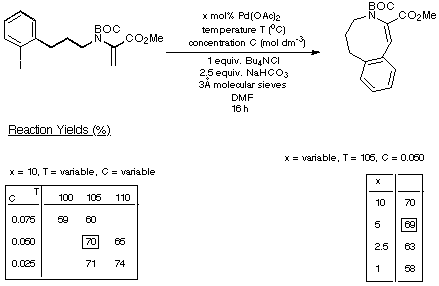
Scheme 16
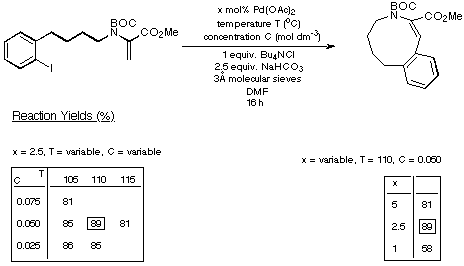
Scheme 17
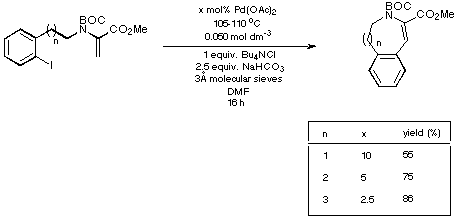
Scheme 18
The figures given in Scheme 18 demonstrate quite clearly that as the
size of the ring being generated increases, the reaction requires less catalyst
and delivers a higher yield of product. Thus the eight- and nine-membered Heck
products are generated in synthetically acceptable yield using acceptable
catalyst loadings.
In view of the moderate yield obtained for the formation of the seven-membered
ring, however, we decided to examine a second approach to the synthesis of the
desired medium-sized rings. Given the nature of the substrates we had in hand,
we chose to determine whether or not radical cyclisations would provide the
required rings in good yield (Scheme 19).

Scheme 19
Once again, a survey of the literature revealed that although radical
cyclisations had been used to generate several hundred five- and six-membered
rings, examples of their use to generate seven-, eight- and nine-membered rings
were relatively rare (Scheme 20).
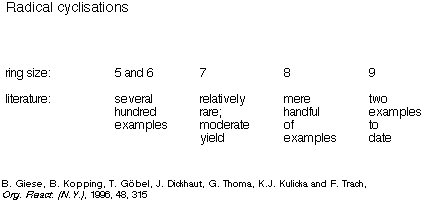
Scheme 20
We were thus gratified to find that treatment of our unsaturated iodoarenes
with Bu3SnH in the presence of AIBN gave the desired cyclised
products in acceptable yield (Scheme 21).
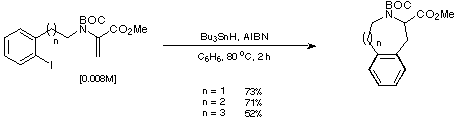
Scheme 21
Comparison of the yields obtained for the intramolecular Heck reaction
and the corresponding radical cyclisations reveal that the two methods are
complementary, the Heck reaction being more favourable for the formation of the
larger rings and the radical reaction providing better yields of the smaller
rings (Scheme 22).
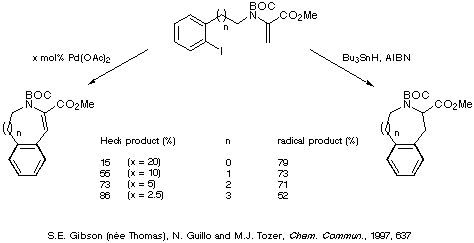
Scheme 22
Once good methods for obtaining the medium-sized rings of Sic, Hic and
Nic were in place, their synthesis was completed in a straightforward manner as
shown in Scheme 23.
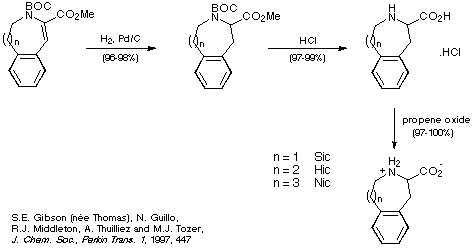
Scheme 23
INCORPORATION OF TIC, SIC, HIC AND NIC INTO A
CHOLECYSTOKININ-B/GASTRIN RECEPTOR ANTAGONIST
The diacid depicted in Scheme 24 is a cholecystokinin-B/gastrin receptor
inhibitor developed by the James Black Foundation.
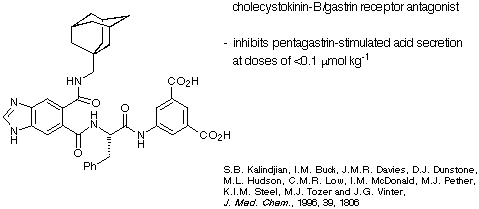
Scheme 24
In order to determine the effect on biological activity of replacing
the phenylalanine residue in the antagonist with Tic, Sic, Hic and Nic and
ultimately perhaps to learn more about the bioactive conformation of the
antagonist, the corresponding Tic, Sic, Hic and Nic analogues were made
according to the route depicted in Scheme 25.
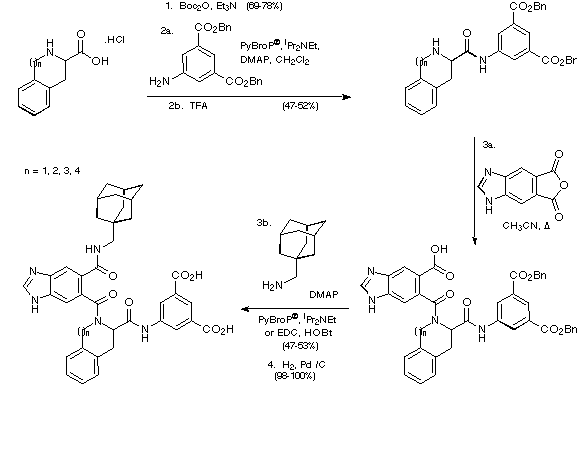
Scheme 25
The results of two biological assays of the parent antagonist, the
four analogues and a commonly used literature reference compound are given in
Scheme 26. These demonstrate that although the Tic, Sic and Hic analogues of
the antagonist 3a-c show much lower binding affinities than the
parent compound 2, compounds 4d containing Nic displayed almost
identical binding characteristics to those of 2. Molecular modelling
studies designed to incorporate this information into the current model of the
bioactive conformation of 2 are now underway.
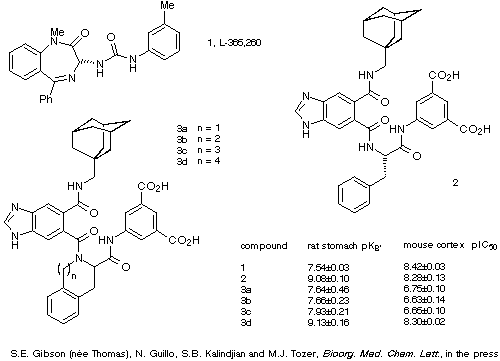
Scheme 26
CONCLUSIONS
We have used transition metal catalysed reactions to synthesise
several new conformationally constrained phenylalanine analogues which together
with the well-established Tic form a series in which the members of steadily
changing conformational freedom. Our first application in a biologically
active system has generated promising results which suggest that the series of
compounds will prove to be considerably more powerful than any one individual
member. We are currently examining further biological applications of Sic, Hic
and Nic.