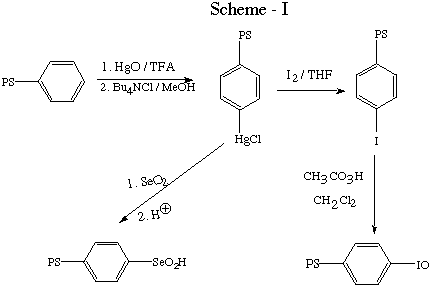
For some time now, our research group has been interested in the
preparation and use of polymer - immobilized reagents. Our initial
work [1,2] centered about the use of divinylbenzene- styrene
copolymers. Eventually we reported on the use of polymer bound
oxidizing agents,[3] principally those involving polymer bound
phenylseleninic acid[4,5] and iodosobenzene[6] (Scheme -I).

We noted polymer degradation with these oxidants, particularly when
using the iodosobenzene reagent. Clearly the benzylic positions are
susceptible to oxidation, thus making styrene-divinylbenzene a less
than optimal choice. As a result, we chose to investigate
fluorocarbon polymers, particularly poly(chlorotrifluoroethylene)
(PCTFE) as oxidation resistant support materials. Our goal has been
to develop methods for replacement of the chlorine with reagent
functionality by substitution. In this fashion, an oxidation
resistant polymer might be obtained.
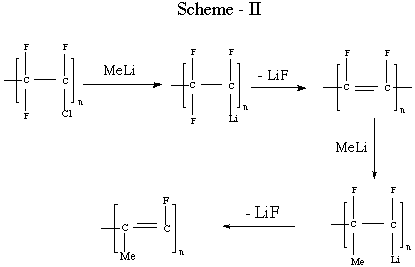
In initial work, we[7] and the others [8-10] looked at the reaction
of PCTFE with alkyl anions. Reactions proceed via metallation and
elimination, followed by further reaction (Scheme - II). McCarthy and
coworker [11] have used this reaction to prepare functionalized forms
of PCTFE and to analyze the high degree of surface selectivity of
such functionalization.
In an important contribution to our work, Cais [12] reported the
conversion of PCTFE to poly(trifluoroethylene) using butyltin
hydride. The high efficiency of this reaction attests to the
stability of the intermediate radical. In the recent past we have
been examining methods to generate and trap this radical, using both
one electron reduction and homolytic cleavage reactions as methods of
generation.
While the strategy of trapping radicals as a means for accomplishing
a substitution is somewhat mature; [13] its application to PCTFE is
somewhat complicated by two factors :
(1) As a solid, PCTFE can remain inert
to a reaction between radical generating agent and trapping agent,
and
(2) The strength of the C - Cl bond
makes cleavage more difficult.
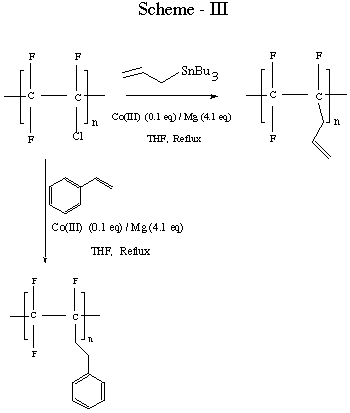
We have reported, [14] along with the others, [15] on the
substitution of chlorine in PCTFE with mercaptide ion (a non surface
- selective reaction). Allylation using heterolytic cleavage or
Co(II) mediated reduction occurs to about a 50% degree of
functionalization (Scheme - III). Limited success was realized in
extending this Co(II) chemistry to other alkylations. [16] In
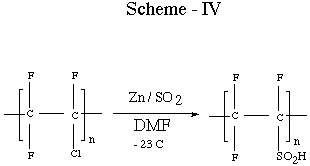
addition the use of Zn / SO2 as a method to
produce sulfinic and sulfonic acid derivative has been carried out
[17] (Scheme IV).
In an intriguing patent report, Lichstein [19] induced transformation
of perfluoroalkylbromides and iodides into carboxylic acid
derivatives. Presumably, the process involves oxidative addition to
the carbon - chlorine bond, carbonyl migration and nucleophilic
addition. At high pressure of CO, the reaction was catalytic in Cr.
We report herein the extension of this chemistry to PCTFE.