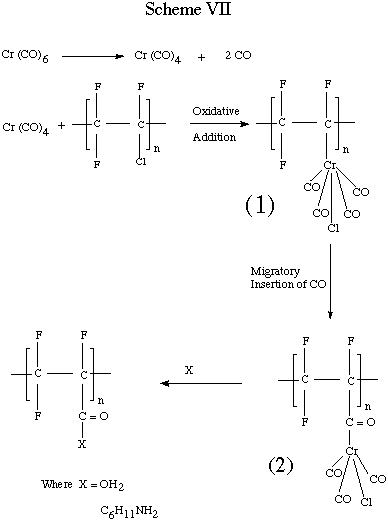
The use of group 6 metal hexacarbonyls provide a route to substitution
of the chloride in PCTFE with acid derivatives. The reaction is not surface
selevctive and is limited to preparing amides and carboxylates. The reaction
mechanism for the reaction may be formulated as follows (Scheme VII):

Reaction of PCTFE with Cr (CO)6 involves oxidative
addition to the carbon - chlorine bond of PCTFE by a coordinatively unsaturated
chromium complex to give the polymer - metal carbonyl complex , (1). The
polymer - metal carbonyl complex then undergoes migratory - insertion of
carbon monoxide to generate the acyl complex , (2). Sebsequent nucleophilic
attack by water or amine gives acid or amide darivative.