Discussion:
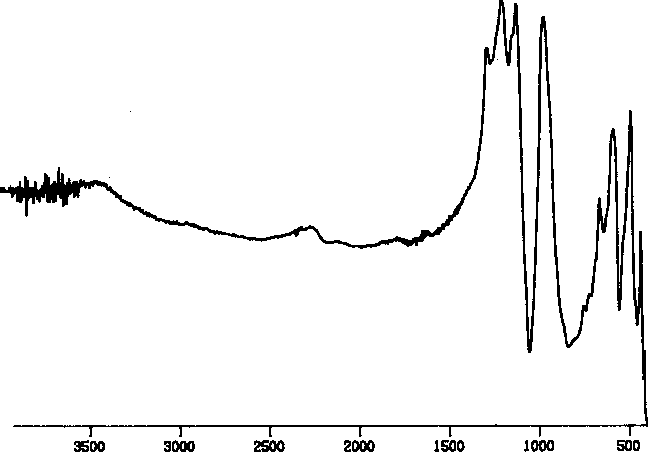
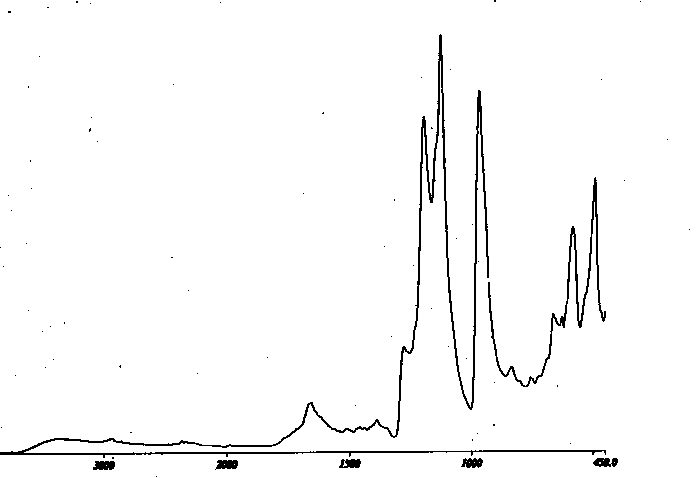
The first screen of the reaction products was carried out using transmission FT - IR. The spectrum of PCTFE (Figure I) clearly shoes the C - Cl streatch at 970 and other peaks for the C - F stretches in the 1100 to 1250 region, with no absorbances in the C - H and C=O regions.

Following this initial screen, the amide product was analyzed by
combustion analysis. In particular, N analysis gives a reasonable
assessment of degree of functionalization. Acid functionalization was
analyzed by titration and electron microscopy, which also provided an
analytical profile for heavy atoms. In this way, a reasonable
assessment of the reaction could be obtained. The best method of
depth profiling available to us in these cases was ATR - FT -IR.
Choice of Metal carbonyl species:
In several contexts, Cr (CO)6 , Mo(CO)6, and W(CO)6 were used in
similar reactions. In no case were significient differences in
results obtained. Metals from other groups (Fe(CO)5, Ru3(CO)12) are far less satisfactory.
Choice of PCTFE:
While little difference in the overall chemistry of Kel - F 6061 and
Neoflon M400H was observed, the degree of functionalization was
dependent on this choice. In general the degree of functionalization
was higher with Kel - F 6061. This is consistent with observation in
previous cases [17] and due to a combination of a somewhat harder
bulk polymer with slighthy larger particle size in the cases of
Neoflon.
Amides:
Reaction of PCTFE with Cr(CO)6 in the presence of cyclohexylamine in DMF at 80C affords a dark product. IR analysis (Figure II) is consistent with formation of the amide as outlined above in Scheme V. Similar results are obtained with other amines such as benzylamine . Since the reactions are analagous, a detailed study of the cyclohexylamine reaction will be described.
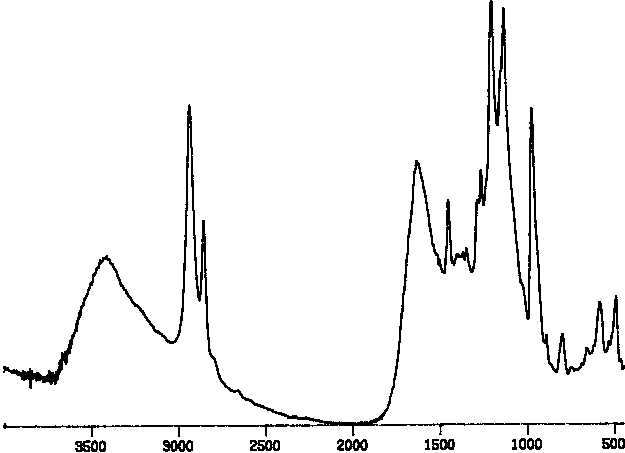
In the IR spectrum (Figure II), the main features to be noted are
the loss of intensity in the C - Cl stretching region (971) and
appearance of new peaks for N -H ( 3390 ), C=O (1700 - 1620 ), and C
-H (2931 and 2885 ). Darkening of the polymer is normally a sign of
elimination related side reactions which are confirmed by the smaller
peaks in the region 1500 -1450. Elemental analysis (C 39.06 %, H 3.66
%, Cl 11.37 %, F 24.45 %, and N 6. 12 %) is consistent with a degree
of functionalization of 84 % , although the extent of side reaction
makes the exact percentage a bit ambiguous.
Acids:
Reaction with either water or alcohols afforded products consistent
with the carboxylate (Scheme VI). In particular the IR spectrum
(Figure III) shows peaks at 3490 for O -H and 1680 for C=O . Once
again, some elimination side reaction can be seen. Calculation using
titrimetric analysis showed the ion exchange capasity for Kel - F and
Neoflon to be 0.39 meq/g and 0.239 meq/g, respectively. This value
seems low in view of the intensity of the C=O peak in the IR
spectrum. It would appear from this analysis that some acid sites do
not participate in ion exchange. Such a phenomenon is not
unprecedented. It is increasingly recognized that so-called "polymer
effects" may significantly change the reactivity and selectivity of
an organic reagent or catalyst once anchored to a polymer
support.[18] The origins of these polymer effects may be physical or
chemical in nature. The various effects are difficult to disentangle
and assess individually, yet the influence of these small but
significant microenvironmental, steric, electronic and intrapolymeric
interactions seems undeniable. An immobilized functionality is in a
very different environment from its homogeneous analog and thus its
activity is altered. In part, the accessibility of the anchored,
reactive sites is affected.
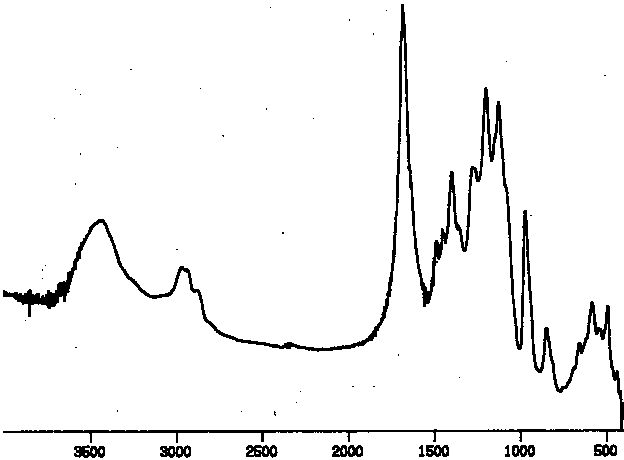
Further insight into the functionalization process is gained from electron microscopy. Comparison of the electron micrographs of Kel - F and Neoflon with their functionalized counterparts shows a significant degree of surface roughening and pitting (Figure IV). Experience associates this phenomenon with the previously noted degradation side reaction. It is also noteworthy that elemental analysis indicated no entrained chromium salts.




Further insight into depth profiling is obtained by ATR - FT -IR (Figures below). The first spectra are that of the Kel-F polymer modified to the carboxylate. While all spectra below are collected at a single bounce reflection of 45 degrees within a hemispherical internal reflection element with an active area 200 micrometers in diameter, the first spectrum (Figure V) uses a germanium internal reflection element and yields a penetration depth of 0.6 micrometers.
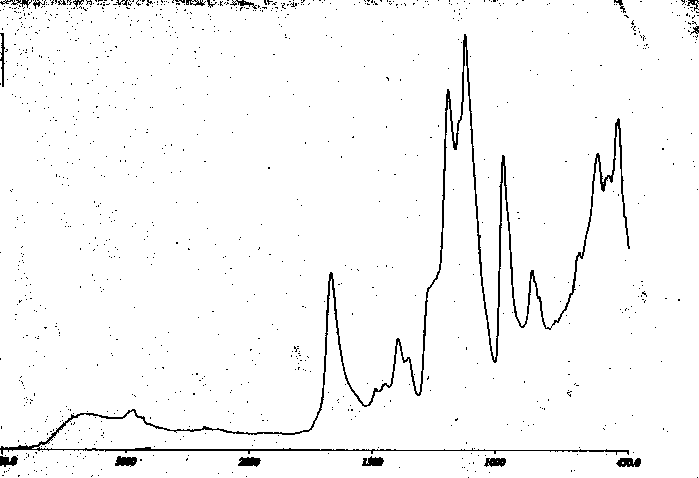
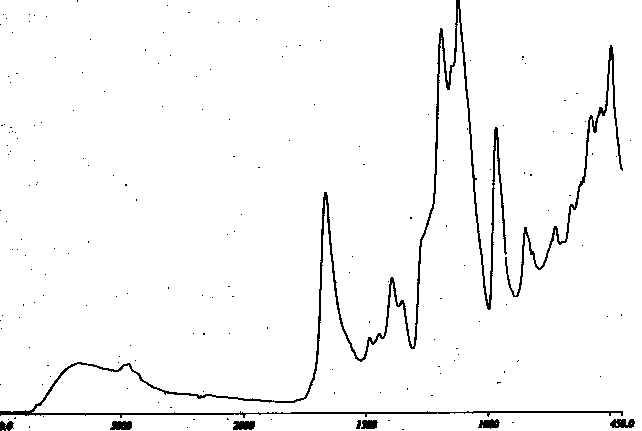
It is useful to compare the spectrum in Figure V, to that of Figure VI, of the same sample using a silicon internal reflectance element and examining a penetration depth of 0.7 micrometers. No evidence for surface selectivity can be seen. Indeed, the deeper penetration seems to exhibit what might be a relatively more intense carbonyl band. This may be attributed to the loss of highly functionalized surface material as fines from the highly etched surface.
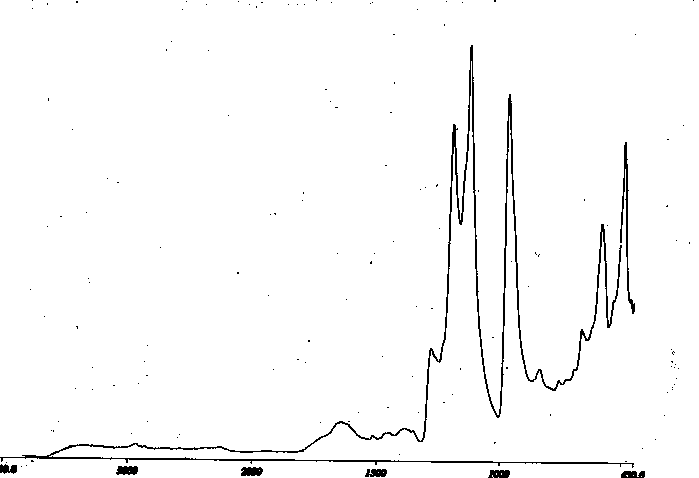
Similar data, although with less loading and a lesser band intensity are seen with the modification of Neoflon. Figure VII shows the germanium internal reflectance sampling and Figure VIII the deeper silicon sampling. Results are analogous.
Such considerations are in accord with our working hypothesis and the low titration values. Other reactions mediated by Cr(CO)6 were attempted. When PCTFE and Cr(CO)6 were reacted with higher boiling alcohols ( isobutyl alcohol, 2 - naphthol, and tert - butyl alcohol), a light brown polymer was resulted. IR analysis in each case conforms the product to be unreacted starting material. The rational for this is steric bulkiness of alcohols, which may reduced accessibility to the reaction sites. This may resulted in failure of the reactions. The reaction of PCTFE and Cr(CO)6 with thiophenol and carboxylic acid were also unsuccessful. The IR analysis of former reaction showed the success of the proposed reaction but with the contamination in the form of metal carbonyl which was indicated by bands located at 1982, 2273, 2338 and 2362.